AtriCure’s Coolrail linear ablation pen for the ablation of cardiac tissue can be used for patients with persistent and ...
March 18, 2008 – Today Bristol-Myers Squibb Medical Imaging introduced its new name by launching Lantheus Medical ...
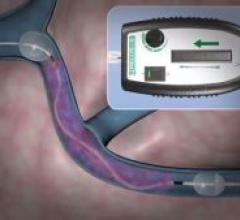
March 18, 2008 - A catheter-based device, the Trellis-8 infusion catheter, which disperses thrombolytic solution ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
McKesson has released a new Horizon Cardiology product for the cath lab, the Holding Area Charting, designed to simplify and expedite information flow in the cath lab, increase data accuracy by minimizing duplication and strengthen the facility’s commitment to patient safety.
March 18, 2008 - Northeast Monitoring has introduced the DR200/HE, a combination 14-day Holter plus 30-day Event ...
Northeast Monitoring has introduced the DR200/HE, a combination 14-day Holter plus 30-day Event recorder integrated into ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 18, 2008 – The FDA gave AtriCure’s Coolrail linear ablation pen 510(k) clearance for the ablation of cardiac ...
Bacchus Vascular Inc. has developed a catheter-based device, the Trellis-8 infusion catheter, which disperses ...
March 18, 2008 – Northeast Monitoring received clearance from the FDA to sell its DR200 Series devices with its ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Agfa will showcase the latest offering of its in Cardiology Data Management, IMPAX CV, focusing on the noninvasive ...
March 11, 2008 - Mindray Medical International Ltd. signed a definitive agreement to acquire Datascope Corp.’s patient ...
March 13, 2008 – Abbott’s fully bioabsorbable drug-eluting stent for the treatment of coronary artery disease ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 11, 2008 – The FDA cleared an investigational device exemption (IDE) application from Life Recovery Systems to use ...
March 18, 2008 - Abiomed Inc. received conditional approval from the FDA to begin its Impella 2.5 Circulatory Support ...
LifeScan Inc. launched two new products to help hospitals improve execution of tight glycemic control protocols - the ...


 March 17, 2008
March 17, 2008