May 7, 2007 — Thoratec Corp., a global maker of products to treat cardiovascular disease, says the FDA has approved an ...
May 3, 2007 — According to a Dow Jones News Service article published by Star Tribune, Minneapolis St. Paul, Boston Scientific Corp. is reviewing its portfolio and will consider selling parts that aren't considered strategic, long-term contributors — so stated the company's chief operating officer Wednesday.
May 3, 2007 — Next Thursday, May 10, in Denver, CO, more than 25 leading heart advocacy groups will announce the formation of the Sudden Cardiac Arrest (SCA) Coalition, dedicated to advancing increased research, awareness and educational efforts to address the nation’s No. 1 cardiovascular killer.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 2, 2007 — Reuters Health reports that scientists have proved that smoking during pregnancy can have lasting ...
May 2, 2007 — Responding to AHA data that estimates 79 million American adults (one in three) have one or more types of ...
May 1, 2007 — Cardiome Pharma Corp. says this week it has received exclusive worldwide rights to Eli Lilly & Co.'s ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
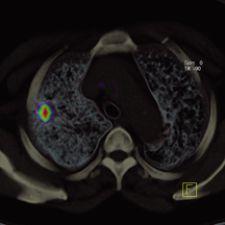
As in most partnerships, rarely are all things equal. Such is the case with hybrid PET/CT systems, where the vast ...
May 1, 2007 — Ventracor has announced the presentation of summary clinical data of the first 100 patients implanted with ...
May 1, 2007 — Acusphere Inc. announced today it exceeded the criteria for success for two of the primary endpoints — ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 1, 2007 — AngioDynamics Inc. announced today the introduction of its Profiler Balloon Dilatation Catheter in the U.S ...
May 1, 2007 — Hansen Medical Inc., a developer of robotic technology for accurate 3D control of catheter movement during ...
Accuray Inc., a manufacturer of radiosurgery devices, has announced it has entered into an agreement with CyberHeart Inc ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 1, 2007 — The Society of Cardiovascular Computed Tomography (SCCT), announced a new annual award program focused on innovation in the field of patient care. The Young Investigator's Award, sponsored by Toshiba America Medical Systems Inc.
April 30, 2007 — Virginia consumers can now find out the latest about how well their local hospitals perform in handling ...
April 30, 2007 — Carotid stenting can be performed on any age patient, but should be undertaken with caution and skill for patients 80 years and older, as well as done safely in experienced centers, according to a study in the May 2007 issue of the Journal of Vascular Surgery.

 May 06, 2007
May 06, 2007




