An implantable technology to control high blood pressure in patients not responding to available medicines has ...
The FDA has cleared Toshiba's new EXCELART Vantage Atlas 1.5T MRI system, which the company showcased this week during ...
An innovative lead insulation material for cardiac leads used with pacemakers and ICDs is now FDA approved and available ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Third International Conference on Cell Therapy for Cardiovascular Diseases will convene Jan 17 to 19, at The New York Academy of Medicine in New York, NY. The three-day program is dedicated to the evolving field of cell-based therapies for cardiac repair and regeneration.
In its third quarter financial report, San Diego-based Cytori said it has moved significantly closer to initiating its cardiovascular clinical trials," said Christopher J. Calhoun, chief executive officer of Cytori Therapeutics. The company is developing adipose stem cells in cardiovascular disease and for use in reconstructive surgery.
Houston based ThromboVision, Inc. has won the first annual Michael E. DeBakey Life Science Award, which carries a $50,000 cash prize to assist in propelling the company's technology toward commercial success.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
November 2006 - Toshiba America Medical Systems, Inc. has announced a new partnership with CVCTA Education and San ...
A German physician reports that people who have implanted pacemakers or defibrillators are not in danger of their ...
The stent market will have a distinctly different look after Johnson & Johnson completes its acquisition of Conor ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Cordis Corp. has received approval from the FDA to market its PRECISE Nitinol Stent and ANGIOGUARD Emboli Capture guide ...
ZOLL Medical Corp. has announced it is now shipping all models of the ZOLL M Series defibrillator that comply with 2005 ...
Designed to speed cardiovascular workflow and help enhance patient care, the Xcelera R2.1 integrates examination results from key cardiology subspecialties, including interventional cardiology, cardiovascular ultrasound, ECG, nuclear cardiology, cardiac CT, cardiac MR and electrophysiology.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
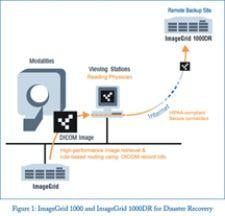
What doesn’t kill us, so the saying goes, makes us stronger. Those of us lucky enough to walk away from tragedy with our ...
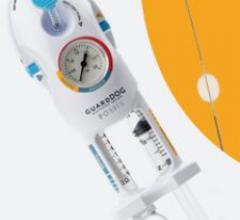
A guide wire-based occlusion device, the GuardDOG by Possis Medical enables physicians to quickly and effectively manage local blood flow while employing interventional techniques and devices to treat vascular disease. It features a 0.035-inch diameter guide wire, preferred for peripheral interventions, and a soft, compliant, CO2-filled balloon providing quick inflation and deflation.
Training physicians, especially cardiac specialists, has taken on an increasingly critical role. With more cardiologists, cardiovascular surgeons and vascular surgeons interpreting CT data, the need for proper training has increased exponentially in recent years.

 November 29, 2006
November 29, 2006