Researchers at the University of California, San Diego School of Medicine have identified a key piece in the complex molecular puzzle underlying heart failure.
Carroll Hospital Center physicians will now have 24/7 remote access to the University of Maryland Medical Center’s (UMMC) Brain Attack Team through a new telemedicine service for stroke patients.
George Adams, M.D., an interventional cardiologist with North Carolina Heart and Vascular, successfully treated a patient having an acute myocardial infarction (AMI) with the Aspire Mechanical Thrombectomy System.
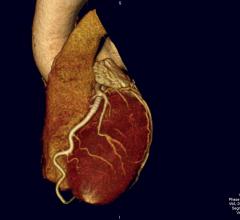
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
In an analysis of outcomes of about 12,000 patients who underwent transcatheter aortic valve replacement (TAVR), death rate after one year was nearly one in four, according to a study in the March 10 issue of JAMA. Of those alive at 12 months, almost half had not been rehospitalized and approximately 25 percent had only one hospitalization.
Carestream is demonstrating its new Touch Ultrasound System at the American Institute of Ultrasound in Medicine (AIUM) annual meeting, March 21-25.
TeraRecon unveiled the latest release of the company’s flagship iNtuition enterprise viewing solutions in combination with the second-generation of its iNteract+ interoperability platform at the annual European Congress of Radiology (ECR) meeting, March 4-8 in Vienna.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
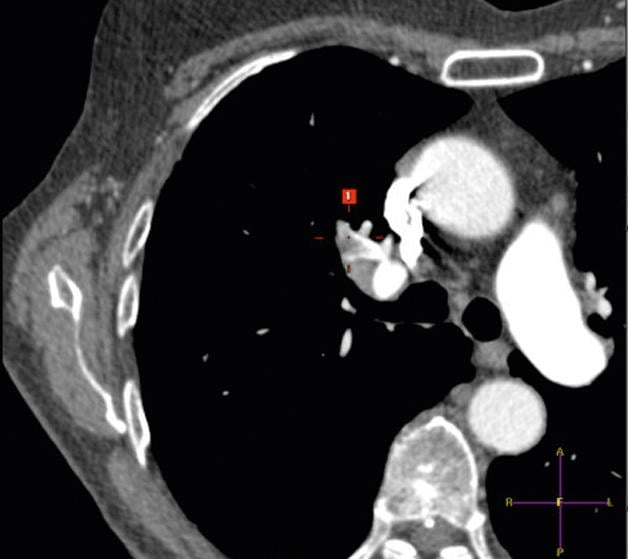
NYU Langone Medical Center has announced the creation of a new multidisciplinary Venous Thromboembolic Disease Center (VTEC) to treat those with life-threatening blood clots. The new VTEC delivers advanced detection, comprehensive care and effective management for patients experiencing a venous thromboembolic event.
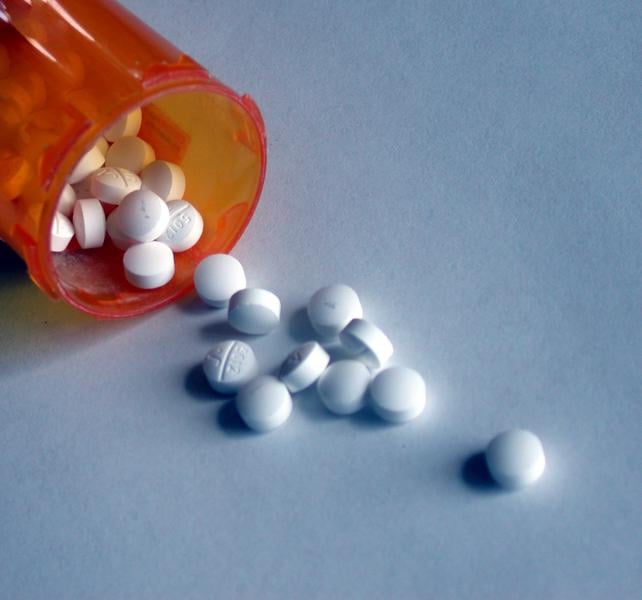
Nearly all women and people over 65 in the United States with atrial fibrillation are advised to take blood thinners under new guidelines based on an analysis from the Duke Clinical Research Institute (DCRI).
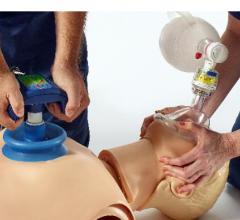
The U.S. Food and Drug Administration (FDA) approved the ResQCPR System, a system of two devices for first responders to use while performing cardiopulmonary resuscitation (CPR) on cardiac arrest patients. The devices may improve the patient’s chances of surviving cardiac arrest.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 9, 2015 — Boston Scientific Corp. announced the settlement of the breach of merger agreement lawsuit brought by ...
Biotronik announced the presentation of data from the iliac arm of the BIOFLEX-I clinical trial at the Cardiovascular Research Technologies (CRT) conference in Washington, D.C. The BIOFLEX-I trial is designed to support U.S. Food and Drug Administration (FDA) approval of the Astron self-expanding nitinol stent to treat patients suffering from common iliac or external iliac artery disease.
AliveCor Inc. announced the launch of the latest version of the AliveECG app, which instantly detects when an electrocardiogram (ECG) is either normal or unreadable.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Fujifilm Medical Systems U.S.A. Inc. will demonstrate the latest version of Synapse Cardiovascular 6.0 and Synapse 3-D 4.1 at the American College of Cardiology (ACC) 64th Annual Scientific Session & Expo on March 14 -16 in San Diego, California.
Corindus Vascular Robotics Inc. announced it has initiated a clinical trial of its CorPath System in peripheral vascular interventions. The study is currently enrolling patients at the Medical University of Graz in Graz, Austria, and is led by Prof. Marianne Brodmann, M.D., a leading researcher with the university’s Division of Angiology, in combination with Prof. Hannes Deutschmann, M.D., of the Medical University of Graz Department of Radiology, and study chairman, Ehtisham Mahmud, M.D., director, Sulpizio Cardiovascular Center-Medicine, UC San Diego.
Bracco Imaging, through its business unit Bracco Injeneering SA, announced the availability of its EmpowerCTA+, an advanced engineering contrast injection system.

 March 10, 2015
March 10, 2015