Primary end-point results from the BIOFLOW-II clinical study demonstrating the non-inferiority of the Biotronik Orsiro Hybrid Drug-Eluting Stent compared to Abbott's Xience Prime. These results were presented in a late-breaking clinical trials session at the EuroPCR congress in Paris by principal investigator, Professor Stephan Windecker, M.D. of University Hospital Bern, Switzerland.
June 28, 2013 — Maquet Cardiovascular LLC announced it has acquired LAAx Inc., a privately held company that has developed a unique mechanical occlusion device called the TigerPaw System II. When implanted, TigerPaw II safely and effectively occludes the left atrial appendage (LAA).
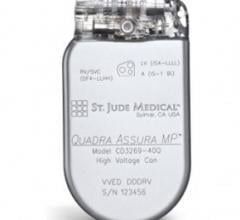
June 28, 2013 — St. Jude Medical Inc. announced CE mark approval of its next-generation quadripolar device, the Quadra Assura MP cardiac resynchronization therapy defibrillator (CRT-D).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Sorin Group announced the results of the DREAM clinical trial. In this study, the sleep apnea monitoring (SAM) algorithm of Sorin Reply 200 pacemakers was validated against polysomnography, the gold standard method used to measure the severity of sleep apnea (SA). Initial data show that Reply 200 with the new SAM algorithm provides reliable screening for the risk of severe SA[1].
Validating its shock reduction research and technology, Medtronic Inc. announced the results of three clinical trials, Shock-Less, PainFree SST and ADVANCE III, which successfully employed key strategies to dramatically reduce inappropriate and unnecessary shocks in patients with implantable cardiac defibrillators (ICD). The studies followed more than 7,000 patients on six continents; results were presented at Heart Rhythm 2013, the Heart Rhythm Society's 34th Annual Scientific Sessions.

Boston Scientific Corporation is launching a new family of pacemakers in Europe. These pacemakers monitor respiration, adjust pacing accordingly, and support insight into the patient's overall heart failure status. Comprising of the Inliven cardiac resynchronization therapy pacemaker (CRT-P) that synchronizes the heart chambers, and the Vitalio and Formio pacing systems, the new family of devices offers clinicians a comprehensive set of tools for the management of heart failure and related comorbidities.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Digisonics will exhibit its No. 1 KLAS rated cardiology picture archive and communications system (PACS) and structured reporting solutions at this year’s American Society of Echocardiography (ASE) Scientific Sessions in Minneapolis, Minn.
June 25, 2013 — Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) has cleared the Virtual Touch imaging ultrasound application, Siemens’ first commercially available implementation of Acoustic Radiation Force Impulse (ARFI) technology.
More than 54 companies will display their latest products and services at the American Society of Echocardiography’s (ASE) 24th Annual Scientific Sessions, planned for June 29-July 2 at the Minneapolis Convention Center in Minneapolis, Minn. This year, the conference theme is “a disease-based focus on the role of echocardiography in diagnosis and guiding therapy”.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 25, 2013 — Zoll Medical Corp. announced that a paper published in Critical Care Medicine reported the likelihood of achieving a return of spontaneous circulation (ROSC) after sudden cardiac arrest (SCA) is 62 percent greater when the AutoPulse noninvasive cardiac support pump is used to deliver CPR chest compressions.
June 25, 2013 — Cordis Corp. announced the European CE marking and U.S. Food and Drug Administration (FDA) approval of additional sizes of its Sleek OTW (over-the-wire) platform, a 0.014-inch ultra-low profile percutaneous transluminal angioplasty (PTA) dilatation catheter.
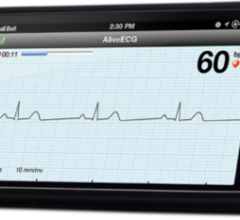
AliveCor Inc.’s Heart Monitor for iPhone 4, 4S and 5 is now available for purchase in the United Kingdom and Ireland. It is the first CE-marked mobile device–based electrocardiogram (ECG) monitor that is used to record, display, store and transfer single-channel ECG rhythms. It is available to medical professionals, patients and health-conscious individuals at Amazon.co.uk.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
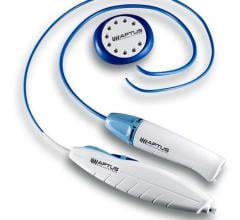
Aptus Endosystems Inc. announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its 28 mm Tip Reach Heli-FX Guide. A line extension of the original Heli-FX System, the new product enhances treatment of wide neck abdominal aortic aneurysms (AAA). The announcement comes ahead of this week’s Society of Vascular Surgery (SVS) Vascular Annual Meeting in San Francisco where the company will be launching this device as well as the recently approved Heli-FX Thoracic System.
Final six and 12-month results of the DIRECT first-in-man clinical study were presented by study principal investigator Dr. Mark Webster at the late-breaking clinical trials session of the EuroPCR meeting in Paris, France. No patients experienced clinically-driven TLR, TVR or MACE at six months, with results sustained through 12 months. It is believed the Svelte drug-eluting stent is the first ever to achieve 0 percent clinically-driven MACE through 12-months in a independent core-lab and DSMB adjudicated clinical study.
The Idaho Health Data Exchange (IHDE) and St. Luke’s Health System (SLHS) have launched Image Exchange viewing capability by eHealthTechnologies.


 July 01, 2013
July 01, 2013