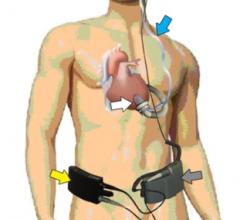
August 17, 2012 — Jarvik Heart Inc., a privately held company that develops and manufactures cardiac assist devices, announced U.S. Food and Drug Administration (FDA) approval of its pivotal trial for evaluation of the Jarvik 2000 heart for destination therapy (DT), named RELIVE (Randomized Evaluation of Long-term Intraventricular VAD [ventricular assist device] Effectiveness).
August 17, 2012 — Siemens Healthcare announced an agreement to enhance the syngo Workflow radiology information system (RIS) by incorporating eXposure, a comprehensive radiation dose management and reporting solution from Toronto-based Radimetrics.
August 15, 2012 — Sound Interventions Inc. has released three-month data from the company's first-in-human clinical study (SOUND-ITV) to treat resistant hypertension through the use of catheter-based ultrasound.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 15, 2012 — Previously unreleased three-year data from the Zilver PTX randomized controlled trial of paclitaxel-eluting stents for femoropopliteal disease indicate that Cook Medical’s paclitaxel-eluting peripheral vascular stent demonstrated 83 percent freedom from TLR at 36 months in the PTX group, compared to 70.2 percent for patients treated with optimal percutaneous transluminal angioplasty and bare metal stents in the 479-patient study.
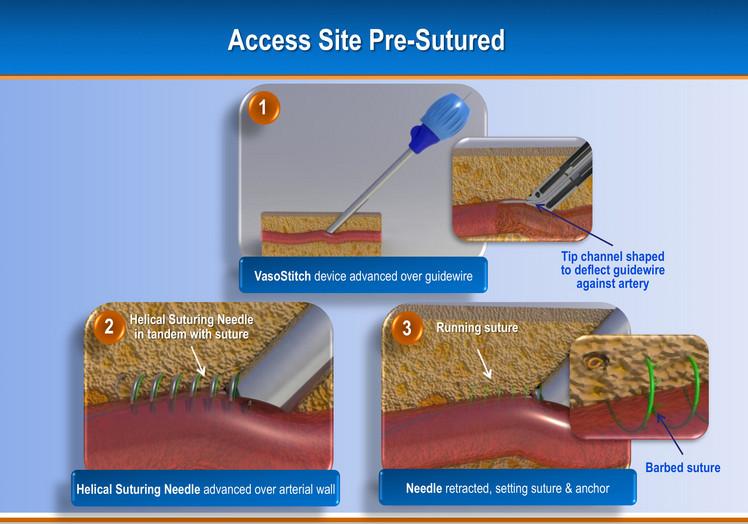
Due to the increasing number of transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR), a new startup company has developed a transfemoral combined access and closure system to accomodate these larger sized percutaneous devices and arteriotomies.
August 14, 2012 — Berlin Heart announced that a study recently published in the New England Journal of Medicine (NEJM) concluded that survival using the Excor pediatric ventricular assist device (VAD) is "significantly greater" than the standard-of-care (extracorporeal membrane oxygenation, or ECMO) as a bridging therapy for children in need of a heart transplant.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
August 14, 2012 — A study led by Kavitha Chinnaiyan, M.D., director of advanced cardiac imaging education, Beaumont Health System (Mich.), has shown that inappropriate CCTA (coronary computed tomography [CT] angiography) use can be reduced by 60 percent with educational programs, increased physician collaboration and close monitoring.
August 14, 2012 — The next five years will see many hospitals in Europe overcoming the high cost and complexity of implementing hybrid operating rooms (OR) by adopting better planning and budget allocation. This will encourage hospitals with larger cardiac and neurosurgery services to implement at least one hybrid OR, suggests new analysis from Frost & Sullivan.
August 14, 2012 — The U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance to Stryker Neurovascular’s Trevo Pro Retriever, indicated for the removal of blood clots. It uses Stentriever technology to optimized clot integration and retrieval in patients experiencing acute ischemic stroke.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
August 14, 2012 — Positron Corp. announced the transfer, consolidation and integration of its radiopharmaceutical operations from the Crown Point, Ind. facility to its Lubbock, Texas site.
Biosense Webster Inc. launched the new Carto 3 MEM (Multi-Electrode Mapping) Version in the United States. It is the latest advancement on the Carto 3 system platform.
August 14, 2012 — The U.S. Food and Drug Administration (FDA) is hosting a public workshop that examines use of absorbable materials in implantable devices for endovascular therapies such as fully absorbable cardiovascular stents, where the stent platform degrades, as well as absorbable coatings.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 13, 2012 — Analog Devices Inc. (ADI) has introduced a low-power, single-lead, heart rate monitor analog front end (AFE) for a wide range of vital sign monitoring applications. The AD8232 AFE is 50 percent smaller and uses up to 20 percent less power than other solutions.

August 13, 2012 — Providers are increasingly focused on using interventional labs in a hybrid operating room (OR) environment, but better support and training are needed to ensure success in this evolution of technology, suggests some of the findings in a recent KLAS study, “Interventional X-ray 2012: A Continuing Evolution.”
August 13, 2012 — To improve magnetic resonance (MR) exam efficiency and image quality, Toshiba America Medical Systems Inc. has received U.S. Food and Drug Administration (FDA) clearance for its high-density 16-element flexible coil system, developed in partnership with NeoCoil. The new coil system makes it easier for clinicians to complete high-quality exams and improve diagnostic efficiency.

 August 17, 2012
August 17, 2012