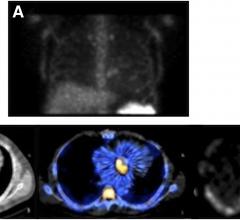
August 9, 2012 — CardioDx Inc. announced that Palmetto GBA, a national contractor that administers Medicare benefits, has established coverage for the company’s Corus CAD gene expression test for the evaluation of patients presenting with typical and atypical symptoms suggestive of coronary artery disease (CAD).
August 9, 2012 — The U.S. Food and Drug Administration (FDA) is informing healthcare providers and patients that the indications for use and labeling for the Stryker Wingspan Stent System have changed to limit the use of Wingspan to a narrow, select group of patients and conditions.
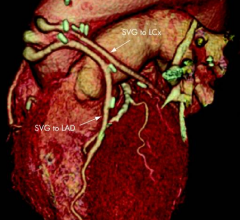
August 9, 2012 — The Harvard Clinical Research Institute (HCRI) announced today the successful completion of randomization in the DAPT study, with the total number of patients randomized exceeding the upper goal set for the study.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Aug. 8, 2012 — The U.S. Food and Drug Administration (FDA) approved the Abbott’s Omnilink Elite Vascular Balloon-Expandable Stent System for the treatment of iliac artery disease. This form of peripheral artery disease (PAD) that affects the lower extremities can progress to where patients experience chronic pain and reduced ability to walk, potentially leading to permanent disability.
Aug. 8, 2012 — Vascular Solutions Inc. launched the SuperCross FT, a new flexible-tip version of its line of SuperCross microcatheters. It is designed to address the majority of complex interventional procedures in which a flexible tipped microcatheter is needed.
Aug. 8, 2012 — The Berlin Heart Group said the U.S. Food and Drug Administration (FDA) has granted approval for its post approval study, a condition of the humanitarian device exemption (HDE) approval that Berlin Heart received for the Excor Pediatric Ventricular Assist Device (VAD) in December 2011.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Aug. 8, 2012 — iHeart Centers acquired the Heart IT WebPAX system for facilitating the management of medical images. The zero-footprint medical image workstation provides Web-based medical image management technology and services to healthcare systems, large hospitals and private clinics.
August 3, 2012 — In a bid to help control healthcare costs, on Oct 1, 2012, the Centers for Medicare and Medicaid Services (CMS) will stop paying hospitals for treating potentially avoidable surgical site infections following cardiac implantable electronic device (CIED) procedures. These include pacemaker and defibrillator implants.
August 3, 2012 — St. Jude Medical recently announced initial findings from the Riata lead evaluation study. The study’s phase I results found that externalized conductors occurred in 9.3 percent of the smaller-diameter Riata ST 7 French leads in the study, and in 24 percent of the larger-diameter Riata 8 French leads.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
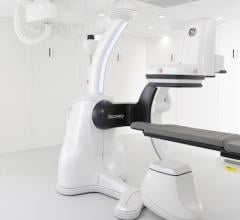
August 3, 2012 — GE Healthcare announced it has already received six orders for its Discovery IGS 730 system since receiving U.S. Food and Drug Administration (FDA) clearance in February 2012.
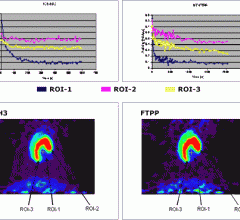
August 3, 2012 — FluoroPharma Medical Inc. announced that they have received high-quality images in an investigator-sponsored clinical trial in China where patients with coronary artery disease (CAD) were given BFPET, its imaging agent for measuring cardiovascular blood flow.
August 2, 2012 — According to new research published in the August issue of the Journal of Nuclear Medicine, single photon emission computed tomography/computed tomography (SPECT/CT) with 99mTc-hexamethylpropleneamine oxime-labeled white blood cells (99mTc-HMPAO-WBC) can improve the diagnosis of infectious endocarditis in hard-to-diagnose cases.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 2, 2012 — Results of the ROMICAT II trial indicates coronary computed tomography (CT) angiography (CCTA) as a safe and time-efficient noninvasive modality to evaluate patients with chest pain in the emergency department (ED) when compared to the current standard approach.
August 2, 2012 — The Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) has released the HRS/ACCF Expert Consensus Statement on Pacemaker Device and Mode Selection, the first of its kind to specifically address pacemaker device and mode selection.

Interventional procedures are growing rapidly and becoming more specialized in diverse areas of care. In the next decade, the number of people living in the United States who have heart conditions is expected to continue to increase, as is the elderly segment of the population and the number of people with chronic disorders such as cardiovascular disease cancer, diabetes and obesity.[1] Driven by these trends and enabled by breakthrough technology allowing new, less invasive approaches to therapy, hospitals are looking at the interventional suite as a strategic area of investment.


 August 09, 2012
August 09, 2012