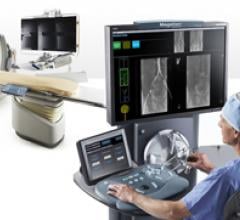
December 28, 2011 — Mindray Medical introduced the DC-T6 ultrasound system at the 2011 Radiological Society of North America meeting.
December 28, 2011 – Good Samaritan Hospital in Los Angeles is participating in the Levant 2 clinical trial to investigate a potential new treatment option for people with peripheral artery disease (PAD). The clinical trial will help doctors determine whether a drug-coated angioplasty balloon will be more successful in keeping narrowed arteries open for longer compared to treatment with a non-coated standard angioplasty balloon.
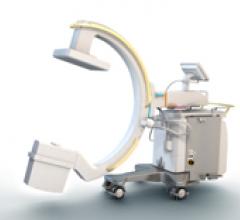
December 28, 2011 — Royal Philips Electronics recently introduced the Philips Veradius Neo mobile C-arm with flat detector. The device allows surgeons to more easily and precisely handle challenging patients and procedures.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
December 28, 2011 – Hansen Medical, a developer of robotic technology for accurate 3-D control of catheter movement, announced the world's first uses of the Magellan robotic system in the treatment of peripheral vascular disease (PVD). The clinical procedures were performed by Professor Nick Cheshire, M.D., and the medical team at St. Mary's Hospital, part of the Imperial College Healthcare in London, pioneers in the use of flexible robotics in vascular interventions.
December 28, 2011 — Quest International Inc. announced the release of the AlphaView AVC2F1P, a wirelessly-enabled color LCD display. The monitor can be used for surgical, operating room (OR), emergency room (ER) and other healthcare environments where viewability, mobility and flexibility are essential. The device is a 24-inch diagonal, 2-megapixel (MP) surgical-grade LCD.
December 27, 2011 — The January 2012 issue of the Journal of the American Society of Echocardiography (JASE) will feature a new resource for guidance on three-dimensional echocardiography (3-DE). The document, titled The EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography, provides a practical guide on how to acquire analyze and display the various cardiac structures using 3-DE.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
December 27, 2011 — ImaCor Inc. announced that 25 leading hospitals in the United States, Canada and Europe are now using its hemodynamic transesophageal echocardiographic (hTEE) management devices. The ClariTEE probe and Zura imaging platform allow physicians to directly visualize cardiac size and function of high-risk anesthesiology patients during and after surgery.
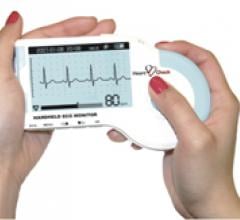
December 27, 2011 — CardioComm Solutions Inc. demonstrated HeartCheck Smart Monitoring, its newly developed remote patient electrocardiogram (ECG) monitoring service, during Medica 2011. The system uses CardioComm’s new application programming interface (API), and their established C4 (CardioComm Coordinating Centre) arrhythmia and ECG management service to interact with handheld patient-activated ECG monitoring devices. Medica 2011 was held in Germany in November.
December 27, 2011 – In a move to re-engineer the process of translating scientific discoveries into new drugs, diagnostics and devices, the National Institutes of Health (NIH) has established the National Center for Advancing Translational Sciences (NCATS). The action was made possible by Congress’ approval of a fiscal year 2012 spending bill and the president’s signing of the bill, which includes the establishment of NCATS with a budget of $575 million.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
December 23, 2011 - Philips Healthcare said Medical City Dallas Hospital, Dallas, Texas, is the first hospital in the United States to use Philips’ HeartNavigator interventional tool in clinical practice. HeartNavigator is a new procedure planning and image guidance tool to help interventional cardiologists and cardiac surgeons perform minimally invasive heart valve replacements. Developed in cooperation with partner hospitals around the world, HeartNavigator is designed to increase the objectivity of the procedure planning and to simplify the procedure itself.
Diagnostic & Interventional Cardiology (DAIC) magazine is looking for readers' opinions about what they think are the most innovative cardiac technologies released in the past year. We also want readers to give us their opinions on what will be the hot trends in 2012.
December 23, 2011 — Biotronik announced the first implantations of the new Lumax 740 implantable cardiac defibrillators — the world’s first and only ICDs eligible for use with magnetic resonance imaging (MRI). The devices — for patients with tachyarrhythmias — feature ProMRI, an MR Conditional feature, combined with an extended device longevity and Biotronik Home Monitoring.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

December 23, 2011 — A new report from KLAS reveals that the cardiology IT market is moving toward consolidation. The report, Cardiology 2012: Will the Complete CVIS Please Stand Up?, explains that as a result of this trend, providers are looking for a technology leader to step up and meet their needs. It examines which vendors that providers feel are poised to lead the cardiology market, provide necessary functionality, and offer integration.
Abbott announced the initiation of ESPRIT I, a first-of-its-kind clinical trial in Europe evaluating the safety and performance of the novel Esprit drug-eluting bioresorbable vascular scaffold (BVS) for the treatment of blockages in the superficial femoral arteries (SFA) and iliac arteries. It will specifically target blockages that resulted in claudication (leg pain upon walking).
December 22, 2011 — TriVascular Inc. announced the first Canadian patients treated with its Ovation Abdominal Stent Graft. The ultra-low profile (14F OD) system is designed to expand the patient population suitable for endovascular aortic repair (EVAR) by addressing a wider range of diseased anatomy. Cherrie Abraham, M.D., vascular surgery, McGill University, performed the first case in Canada at Montreal Jewish General Hospital (JGH). The second case, also performed at JGH, was performed by Daniel Obrand, M.D., chief of vascular surgery, JGH.

 December 28, 2011
December 28, 2011