November 4, 2011 — The U.S. Food and Drug Administration (FDA) has approved a stent graft system for patients with small arteries; it will give them the option of less invasive surgery to repair their potentially life-threatening abdominal aortic aneurysm.
An abdominal aortic aneurysm is a bulge in the part of the aorta that runs through the abdomen and divides into the arteries that supply blood to each leg. Over time, this bulge can become weak, and the force of normal blood pressure can cause it to rupture, which can be fatal.
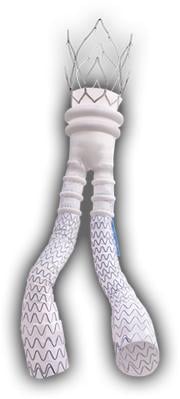
Aneurysms can be repaired through open surgery or less invasively with endograft repair using a stent graft. Treatment with a stent graft depends on the patient's anatomy. A small number of people have blood vessels that are too small in diameter to accommodate typical endograft systems on the market.
The Ovation Abdominal Stent Graft System (20 millimeter diameter), manufactured by TriVascular Inc., is not only small in diameter but it uses a narrower delivery system than any other marketed endograft — 4.7 mm in diameter compared to 7 mm in diameter for typical delivery catheters.
"The unique construction of the 20 mm Ovation system allows it to fit into a narrower delivery catheter than other endografts currently on the market. FDA's approval of this product will enable some patients with small blood vessels to be treated via minimally-invasive surgery who did not previously have this option," said Christy Foreman, director of the Office of Device Evaluation in the FDA's Center for Devices and Radiological Health.
Approved by the FDA on Nov. 1, the system differs from traditional endografts in that a portion of the metal stent is replaced with ring-shaped channels. After the device is in place in the aorta, the channels are injected with a polymer, expanding the endograft against the aorta to create a seal.
The system was approved as a Humanitarian Use Device (HUD), an approval pathway limited to devices that treat or diagnose fewer than 4,000 people in the United States annually. To obtain approval for humanitarian use, a company must demonstrate a reasonable assurance the device is safe and that its probable benefit outweighs the risk of illness or injury. The company also must show there is no comparable device available to treat or diagnose the disease or condition.
The FDA reviewed clinical data from a study of the Ovation system, in which four participants were treated with the 20 mm device. The data were considered relevant in evaluating safety and probable benefit for the HUD.
Investigators monitored participants and conducted imaging studies at one, six, and 12 months; they were looking for complications such as movement or kinking of the endograft, leaks in the seal between the endograft and the aorta, and aortic ruptures.
The FDA also considered data from a study of an earlier version of the Ovation that supported the stent graft's long-term ability to seal off an abdominal aortic aneurysm.
Serious adverse events were consistent with other endovascular grafts and included blood and lymphatic problems, cardiac events, gastrointestinal problems, genitourinary problems, and pulmonary problems.
Investigators will continue to track participants for complications and adverse events for five years.
The FDA approved the Ovation system for use in patients with iliac or femoral artery access of fewer than 7 mm and an aorta with an inner diameter between 15.5 mm and 17.4 mm. The system is contraindicated for use in patients with an infection that threatens to infect the graft and in patients who have allergies to the device materials. It should not be used in patients who are unable to undergo the necessary preoperative and postoperative imaging studies.
For more information: www.fda.gov


 September 18, 2025
September 18, 2025 









