March 28, 2011 – Patients who have suffered a "mini stroke" are at twice the risk of heart attack than the general population, according to research reported in Stroke: Journal of the American Heart Association.
March 28, 2011 – The U.S. Food and Drug Administration (FDA) has approved two new cardiac resynchronization therapy-pacemaker (CRT-P) systems. The Consulta and Syncra CRT-P systems, from Medtronic, are the first to include OptiVol Fluid Status Monitoring. The feature identifies patients at risk for worsening heart failure before symptoms develop.
March 28, 2011 – The Society of Nuclear Medicine’s (SNM) 2011 Annual Meeting, June 4-8 in San Antonio, offers a variety of new education options to nuclear medicine and molecular imaging professionals. With new topics, formats and technologies, the meeting provides a comprehensive review of the latest research and issues in the field.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Horizon Cardiology, a cardiovascular information system (CVIS) from McKesson, is a comprehensive information solution from fetal to adult (including congenital) that supports cardiac and peripheral catheterization, hemodynamic monitoring, echocardiography, vascular ultrasound, nuclear cardiology and electrocardiogram (ECG) management.
Horizon Cardiology, a cardiovascular information system (CVIS) from McKesson, is a comprehensive information solution from fetal to adult (including congenital) that supports cardiac and peripheral catheterization, hemodynamic monitoring, echocardiography, vascular ultrasound, nuclear cardiology and electrocardiogram (ECG) management.
March 25, 2011 –At Jersey Shore University Medical Center, 66-year-old heart attack survivor Charles Theodora from Ocean Township became the first N.Y./N.J. metropolitan resident to be implanted with an experimental cardiac monitor system. The AngelMed Guardian cardiac monitor and alert system, by Angel Medical Systems, can alert Theodora if a heart attack is imminent.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 25, 2011 – A new galectin-3 test, recently cleared by the U.S. Food and Drug Administration (FDA) to help assess the prognosis of patients with chronic heart failure, is now offered by Health Diagnostic Laboratory (HDL). BG Medicine Inc. announced the agreement under which HDL will offer galectin-3 testing services based on the BG Medicine Galectin-3 Test.
March 25, 2011 – The American College of Cardiology (ACC) and the American Heart Association (AHA) have released a focused update to the 2007 guidelines for the managing patients with unstable angina (UA)/non-ST-elevation myocardial infarction (NSTEMI).
March 25, 2011 – A new, next-generation aspiration catheter has been introduced in the United States. The Fetch 2 Catheter, by Medrad Interventional, features a flexible, variable pitch coiled shaft for improved kink resistance.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
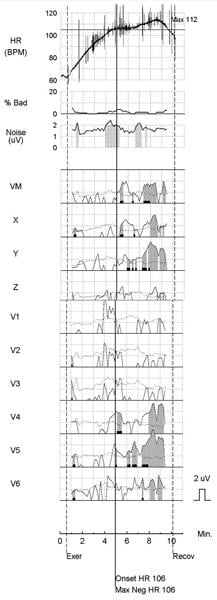
During a sudden cardiac arrest (SCA), heart function ceases – abruptly and without warning – most often due to the onset of a ventricular tachyarrhythmia [1]. Because out-of-hospital survival is less than 8 percent, prediction and prevention are critically important [2].
March 24, 2011 – Infinitt North America and Intelligent Business Solutions (IBS) announced a marketing partnership to enable a single point-of-access to both the CardioPulse Cardiovascular Information System (CVIS) and Infinitt Cardiology Picture Archiving and Communications System (PACS) functionality.
March 24, 2011 - The first implant in the United Kingdom of the Omega Platinum Chromium Bare-Metal Coronary Stent System was announced by Boston Scientific. The stent recently received CE mark approval. The first implant was performed by Dr. Neal Uren, consultant cardiologist, Royal Infirmary of Edinburgh.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 23, 2011 - Millar Instruments Inc. is issuing a worldwide recall of 1,080 units of its Human Use High Injection Angiographic Catheter because they may contain particulate debris within the catheter lumen.
March 22, 2011 – The U.S. Food and Drug Administration (FDA) has approved a 210-patient investigation devices exemption (IDE) clinical trial to test the efficacy of a bone marrow stem cell technology to treat patients with non-reconstructable critical limb ischemia (CLI).
The U.S. Food and Drug Administration (FDA) has granted InfiMed 510(k) market clearance for its i5 Nexus-DRF multi-purpose digital imaging system. The new i5 product line combines radiographic fluoroscopy (RF), digital radiography (DR) and cardiac capabilities on one imaging platform using various industry-leading flat panel detectors.

 March 28, 2011
March 28, 2011