March 29, 2010 - Medical industry leaders gather this week to discuss steps manufacturers of CT and fluoroscopy systems can take to reduce unnecessary patient exposure to ionizing radiation.
March 29, 2010 – A new multiparameter ambulatory monitoring solution that transmits electrocardiogram (ECG) and other clinical modalities via cellular network, enables real-time data analysis for more expedient triaging of critical patients. ScottCare Corp. just launched TeleSentry, the latest innovation to its CardioView Dx suite of cardiovascular diagnostic solutions.
March 24, 2010 — Several genes are associated with heart disease, stroke, and high blood pressure, and can determine whether people may have predisposition to these conditions, according to genetics research.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 26, 2010 – The first implantations were announced yesterday for a single-chamber implantable cardioverter defibrillator. The system provides physicians with complete diagnostic information not only from the right ventricle, as in regular single-chamber ICDs, but also from the right atrium, including high-resolution online IEGMs.
March 26, 2010 — The American College of Cardiology (ACC) and Heart Rhythm Society (HRS) recently announced a new collaboration to build and deliver innovative programs and resources for cardiology clinicians treating patients with atrial fibrillation (AF).
March 26, 2010 – The FDA granted 510(k) market clearance for the AngioSculpt PTA Scoring Balloon Catheter for dilatation of lesions in renal arteries.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 26, 2010 – Nearly 14 percent of Medicare beneficiaries admitted with a primary diagnosis of ST-elevated myocardial infarction (STEMI) undergo multivessel primary percutaneous coronary intervention (PCI), despite recommendations from the American College of Cardiology and the American Heart Association to intervene on only the culprit vessel.
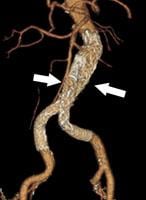
March 26, 2010 – When actor John Ritter died suddenly in 2003 from a tear in his thoracic aorta, the tragedy brought attention to a rare but deadly condition that takes the lives of an estimated 10,000 Americans each year.
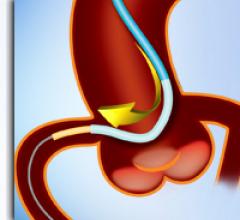
March 25, 2010 – The FDA this week granted permission for a conditional investigational device exemption (IDE) to evaluate the safety and efficacy of a transcatheter plug to seal the left atrial appendage (LAA). The appendage is frequently the source of stroke-causing clots in atrial fibrillation patients.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 25, 2010 – Recognizing that a single database cardiovascular information system(CVIS) can streamline workflow and improve staff efficiency in the cardiology department, several hospitals are replacing their legacy hemodynamic systems with a unified CVIS.
March 25, 2010 – The Transradial University Web site was launched this week to help expand the use of transradial access as an alternative vascular access site. As transradial adoption expands in the U.S., two key limiting factors are training and experience.

Final five-year results from the ENDEAVOR III trial, comparing the Endeavor Zotarolimus-Eluting Coronary Stent to the Cypher Sirolimus-Eluting Coronary Stent, showed Endeavor had lower long-term rates of adverse events, cardiovascular death and heart attacks. The results were released last week during the American College of Cardiology’s 59th Scientific Session.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The GuideLiner catheter is a coaxial “mother and child” guide extension using a rapid exchange system that provides back-up support and selective deep intubation in challenging coronary interventions. The catheter is available in 6, 7 and 8 French sizes.
A new and improved design of the original Gopher catheter, the Gopher Gold is designed for use when treating coronary and peripheral stenoses over an existing in-place 0.014-inch guide wire.
The Merit Laureate hydrophilic guide wire is designed for drainage catheter access, dialysis catheter placement, as well as difficult vascular access procedures.


 March 29, 2010
March 29, 2010