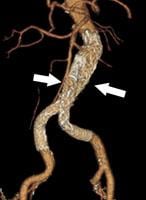
March 26, 2010 – When actor John Ritter died suddenly in 2003 from a tear in his thoracic aorta, the tragedy brought attention to a rare but deadly condition that takes the lives of an estimated 10,000 Americans each year.
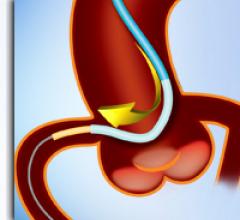
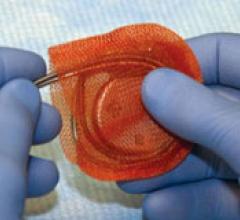
March 25, 2010 – The FDA this week granted permission for a conditional investigational device exemption (IDE) to evaluate the safety and efficacy of a transcatheter plug to seal the left atrial appendage (LAA). The appendage is frequently the source of stroke-causing clots in atrial fibrillation patients.
March 25, 2010 – Recognizing that a single database cardiovascular information system(CVIS) can streamline workflow and improve staff efficiency in the cardiology department, several hospitals are replacing their legacy hemodynamic systems with a unified CVIS.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 25, 2010 – The Transradial University Web site was launched this week to help expand the use of transradial access as an alternative vascular access site. As transradial adoption expands in the U.S., two key limiting factors are training and experience.

Final five-year results from the ENDEAVOR III trial, comparing the Endeavor Zotarolimus-Eluting Coronary Stent to the Cypher Sirolimus-Eluting Coronary Stent, showed Endeavor had lower long-term rates of adverse events, cardiovascular death and heart attacks. The results were released last week during the American College of Cardiology’s 59th Scientific Session.
The GuideLiner catheter is a coaxial “mother and child” guide extension using a rapid exchange system that provides back-up support and selective deep intubation in challenging coronary interventions. The catheter is available in 6, 7 and 8 French sizes.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A new and improved design of the original Gopher catheter, the Gopher Gold is designed for use when treating coronary and peripheral stenoses over an existing in-place 0.014-inch guide wire.
The Merit Laureate hydrophilic guide wire is designed for drainage catheter access, dialysis catheter placement, as well as difficult vascular access procedures.
March 24, 2010 – A team of Mayo Clinic researchers found that cardiac rehabilitation is associated with significantly reduced mortality rates for patients who receive coronary stents. The findings were presented last week at the annual meeting of the American College of Cardiology in Atlanta.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 24, 2010 – Enrollment began this week in the CITADEL, which is the second of two large-scale, prospective, multicenter studies comparing implantable electrophysiology device infection rates. The study will look at patients with and without a new anti-bacterial envelope covering their devices.
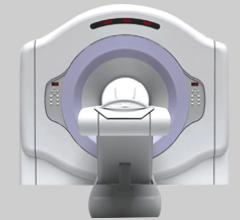
March 23, 2010 – Frost and Sullivan gave a 2010 North American New Product Innovation Award to Positron Corp. for its pioneering cardiac positron emission tomography (PET) scanner, the Attrius.
March 24, 2010 – The 2010 Annual Meeting of the American Association for Thoracic Surgery (AATS) will showcase two multipurpose interventional operating room suites that integrate digital imaging diagnostics, catheterization, and surgical capabilities.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

March 18, 2010 – The U.S. Food and Drug Administration (FDA) today added a boxed warning to the anti-blood clotting drug Plavix (clopidogrel), alerting patients and healthcare professionals that the drug can be less effective in people who cannot metabolize the drug to convert it to its active form.
March 23, 2010 – The U.S. Food and Drug Administration (FDA) recently added a boxed warning to the anti-blood clotting drug Plavix (clopidogrel), alerting patients and healthcare professionals that the drug can be less effective in people who cannot metabolize the drug to convert it to its active form.
March 18, 2010 — A cardiac cryoablation catheter system designed for patients with paroxysmal atrial fibrillation (PAF) has completed premarket approval and is under review by the U.S. Food and Drug Administration (FDA).

 March 26, 2010
March 26, 2010