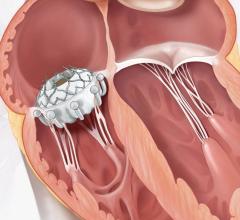
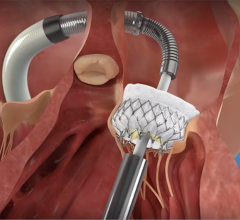
(This article was updated with new information in June 2016) Transcatheter structural heart repair devices deployed in the cath lab have largely replaced open-heart surgery as the therapy of choice. Percutaneously deployed septal occluders make it possible to repair atrial septal defects with better outcomes, fewer complications, faster patient recovery and lower health care costs.
Congenital heart disease was once limited to pediatrics. However, advances in medicine over the past 30 years have led to increasing numbers of children growing up to be adults with these conditions, which are now being treated later in life.
“There are now more adults with congenital heart disease than children, and that number is growing,” said David Balzer, M.D., director, cardiac catheterization laboratory, St. Louis Children’s Hospital, and associate professor of pediatrics at Washington University in St. Louis.
This fact, combined with transcatheter occluder technology, is leading many mainstream cardiologists to begin treating these defects, Balzer said.
Watch the VIDEO "Transcatheter Closure of Holes in the Heart." Zayad Hijazi, M.D., MPH, MSCAI, FACC, director of the cardiac program and chair of the Department of Pediatrics at Sidra Medical and Research Center in Doha, Qatar, discusses the latest technologies for ASD and PFO closure at ACC.17.
Video animation of a transcatheter PFO occluder implantation.
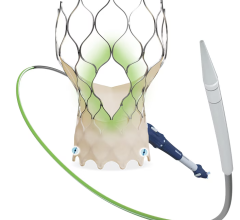
Transcatheter Septal Occluders
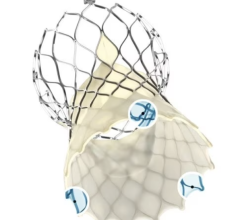
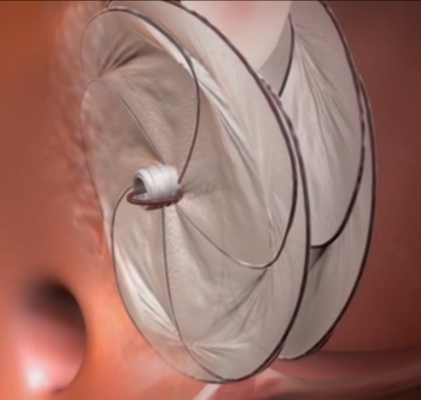
There are only two approved transcatheter septal occluders on the U.S. market. Both are cleared to treat atrial septal defects (ASDs), but are frequently used off-label to treat patent foramen ovales (PFO). The AGA Amplatzer became the first, with U.S. Food and Drug Administration (FDA) clearance in 2001. It is constructed of a nitinol wire mesh that opens up into a round plug to seal the hole. St. Jude Medical purchased AGA in 2010.
W. L. Gore & Associates developed the Gore Helex Septal Occluder, which gained FDA clearance in 2006. The device consists of a corkscrew-like nitinol wire frame with a Gore-Tex material covering. The biocompatible material allows progressive tissue growth to help seal the defect.
Gore is seeking expansion of the Helex indication to treat PFOs. In December 2008, the Helex began an FDA-approved clinical study to demonstrate the safety and effectiveness of the device for PFOs. AGA also developed a new device specifically for PFOs that is not yet FDA cleared.
In May 2016, an FDA panel voted to recommend approval of the Amplatzer PFO Occluder System to treat cryptogenic stroke, but had panel members had some reservations about the device and the votes were not unanimous. Read the article PFO Occluder for Cryptogenic Stroke Recommended by FDA Panel. The FDA cleared the Amplazer system for PFOs in October 2016
These are life-long implants, and long-term outcomes are very good, said Ziyad M. Hijazi, M.D., MPH, professor of pediatrics and internal medicine, director of the Rush Center for Congenital and Structural Heart Disease at Rush University Medical Center, Chicago. “The endothelial tissue grows over the device and it becomes a part of you,” Hijazi said.
He is working on a paper about the long-term outcomes of patients who received these devices, including some of the first who received the Amplatzer in the mid-1990s.
In 2015, Gore released a replacement device for the Helex — the Cardioform Septal Occluder for the transcatheter closure of ASDs and PFOs. It is a permanent implant consisting of a wire frame covered with a thin ePTFE membrane. The wire frame is made of a nitinol alloy. It was first shown at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting.
A Revolution in Structural Heart Repair
Hijazi has been involved with structural heart repair devices since he was a fellow at Yale in 1988. He worked with the Clamshell device prior to the FDA withdrawing it from the market due to frame fractures. In 1995, he served as principal investigator for the trial of the Das-Angel Wings device. In 1997, he became principal investigator for the AGA Amplatzer occluder, which he saw through until its FDA approval in 2001.
“The introduction of the Amplazter device was the turning point. It created a revolution in structural heart repair,” Hijazi said.
He explained all previous transcatheter occluders were complicated and difficult to use. “Now with the Amplatzer, every Tom, Dick and Harry can do septal repairs,” Hijazi said.
The ease of use has caused the number of surgical repairs of PFOs and ASDs to drop by at least 75 percent, he said. At Rush, 95 percent of all ASD and PFO repairs are now done transcatheter.
Ease of Use
“It seems like every interventional cardiologist says they do PFO closures now,” Hijazi said. “The training is easy and the device is so easy to use, so everyone can do it.” He believes the majority of transcatheter PFO and ASD repairs today are performed in standard interventional cath labs.
The training to use these devices includes a day of simulation training and lectures with the manufacturer. This is followed by performing a couple of cases with a proctor.
“About 80 percent of these cases are as easy as pie,” Balzer said, adding it is the remaining 20 percent that present problems for inexperienced operators. “For large ASDs or those with deficient rims, there is a definite learning curve. Not all of these cases are a slam-dunk.”
Procedural Complications
Hijazi said many physicians doing PFO and ASD closures are low-volume practitioners who only do a few cases a year, which is not enough to make them experts. While the basic procedure is easy to perform, inexperienced physicians can run into unexpected complications.
“I get phone calls every week from cardiologists with a patient in the cath lab,” Hijazi said.
The most common complication is embolization, where the device is not seated properly and dislodges. Hijazi said it usually goes into the pulmonary circulation and occasionally ends up in the aorta.
“The overall complication rate should be very low,” Balzer said. Embolization only occurs in about 1 percent of patients, he explained. “You can usually remove it in the cath lab, but some patients will need surgery.”
A less common complication is tissue erosion around the occluder, usually caused by an oversized device, for example, repairing a 1 cm hole with a 2.5 cm device. Hijazi said device sizing is critical, otherwise the large tissue overlap of the metal frame can file away at the tissue with the heart’s constant motion. Erosion requires open-heart surgery to repair. If left untreated, he said the device might cut its way through the wall of the heart. “That can be catastrophic or even fatal for the patient, but fortunately the risk is very low,” Balzer said.
Read article on FDA safety warning regarding Amplatzer erosion reports
The Pros and Cons of the Devices
The Helex only has one wire and is coated with a soft material, so it is much gentler and less abrasive than the exposed, woven nitinol wire framework of the Amplatzer, Hijazi said. “I use the Helex device almost exclusively for all PFOs,” Hijazi told Diagnostic and Invasive Cardiology. At Rush, about 95 percent of PFOs and 70 percent of ASDs are now treated with the Helex device.
To Balzer’s knowledge, there have not been any reported cases of erosion with the Helex.
While the Helex appears to be a reasonable choice for the majority of smaller holes, the Amplatzer appears to be better when treating large holes. Hijazi said its nitinol framework offers structural strength across a larger area.
Balzer added the single wire of the Helex can fracture, especially in the larger devices. For this reason, he uses the Amplatzer for larger holes.
Indications for use
“Probably 90 to 95 percent of ASDs are closed in the cath lab, but there are still some cases that require surgical closure because they are too large or because of the tissue rim,” Balzer said.
Occluders cannot fix all holes, Balzer said. Ostium primum ASDs, occurring in the lower part of the septum near the mitral valve, require surgical repair. He said the location of the hole does not provide enough space for the occluder without it interfering with the function of the valve.
“Not every patient is qualified for these devices,” Hijazi said. “They need to have rims around the hole to help capture the device.”
Generally, Balzer said you need about 5 mm of tissue along the rim to properly anchor these devices. He also warns that while the devices are circular, the holes frequently
are not.
Advice to Physicians
For interventional cardiologists who want to treat these structural heart cases, the three key pieces of advice are collaboration with other physicians, training and sizing.
“If they have the ability to coordinate with their pediatric colleagues, it is very helpful, because they can draw on their experience and expertise with these procedures,” Balzer said.
“Train, train, train, attend courses and ask for help — don’t be a cowboy. Also, work on increasing your volume,” Hijazi suggested.
The larger the hole, the more challenging it is to treat, which is why Hijazi suggested novice physicians only treat smaller defects until they gain more experience. “I can tackle any size hole you give me, but I have the experience to treat it,” he said. “A small ASD is a slam-dunk for anyone, but a large ASD is not so easy.”
Proper sizing is also key. Balzer uses ultrasound to image blood flow through the shunt while inflating a compliant balloon in the hole. When the flow stops, the balloon will indicate the size of the
device required.
Treating VSDs
The most difficult holes in the heart to repair are ventricular septal defects (VSDs). They are much harder to reach by catheters alone. The best method to reach VSDs is using a minimally invasive surgical approach in a hybrid operating room, Hijazi said.
NTM Medical’s CardioSeal and StarFlex have FDA clearance for muscular VSD repairs, as does the Amplatzer muscular VSD device. The StarFlex is currently in U.S. trials to test its safety and efficacy in treating PFOs.
Read the related 2017 article “PFO Closure Shows Positive Results from REDUCE Clinical Study.”



 July 08, 2024
July 08, 2024