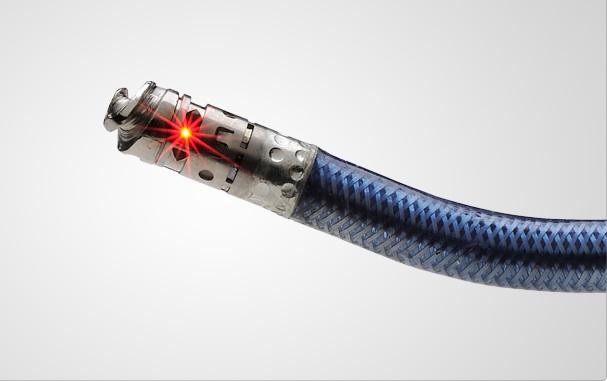
Avinger gained CE mark for a forward-looking optical coherence tomography (OCT) system to aid recanalization of chronic total occlusions (CTOs).
Each year that I attend Transcatheter Cardiovascular Therapeutics (TCT), I comb the event and its sessions looking for the next big trend or technological innovations in cardiovascular devices. Below are my finds for the most cutting-edge and futuristic technologies at TCT 2011.
Robots in the Cath Lab
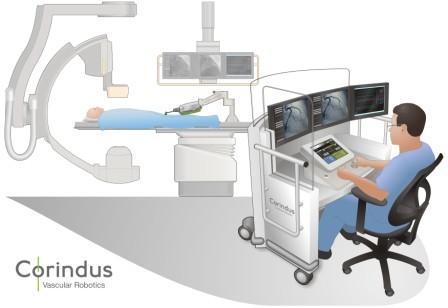
Interest has exploded over the past year in robotic cath lab guidance technology since Corindus began its U.S. Food and Drug Administration (FDA) pivotal trial. The Corindus robotic-assisted PCI system allows operators to sit in a lead-lined booth to manipulate catheters by joystick, rather than wearing lead aprons and standing in or near the X-ray field.
I sat in on a breakfast session about the system presented by interventional cardiologists Giora Weisz, Ron Caputo, Jeff Moses, Chris Metzger and Peter Fitzgerald. All of the speakers have “drunk the Kool-Aid,” so to speak, and praised the system’s capabilities. All agreed the system worked much better than they ever anticipated and will likely become the future for cath labs. They all said the system enables easier catheter navigation because of more precise catheter manipulation and prevention of wire “slippage.” Easier vessel navigation also means less radiation for patients, and removing the physician from the radiation field results in a massive cut in their radiation exposure.
“We measured radiation exposure and there was a 97 percent reduction in exposure, without wearing a lead apron,” Weisz said.
In addition, the system allows operators to perform their work without standing while wearing lead aprons, helping to prevent the common orthopedic issues many interventionalists face.
Hanson Medical, which has already commercialized a robotic electrophysiology (EP) catheter guidance system, has also developed a robotic peripheral vascular interventional guidance system called Magellan. The system is 510(k) pending and is not yet available in the United States.
Interactive Holograms to Visualize Complex Anatomy
The limits of treating 3-D anatomy using 2-D imaging are well known, but this constraint may soon be overcome with new technology developed by RealView Imaging. The company created a system that projects true 3-D holograms that can be displayed mid-air, under normal lighting conditions and without the use of special glasses. Instead of a 3-D rendering from a computed tomography (CT) scan or rotational angiography being displayed on a flat 2-D screen, the holograms can be projected in actual 3-D over a patient in the cath lab.
The images can be rotated by the operator by moving it with his fingers or sliced through using a hand-held wand. Developers say the 3-D model will allow operators to hold up devices to see how they fit within the anatomical image for better sizing.
“This is not science fiction – this exists,” said Elchanan Bruckheimer, MBBS, Scheider Children’s Medical Center, Petach, Israel, who is working with the company. “Surgeons work in 3-D all the time, but as an interventionalist, you are working through a very tiny hole. This technology allows you to see the anatomy in 3-D and to interact with the anatomy…We want to simplify what are becoming very complicated procedures.”
Transcatheter Pacemakers
In a presentation about next-generation pacing technologies, Stephen Oesterle, senior vice president for medicine and technology, Medtronic, said the Achilles heel of pacing devices today is in their fragile leads. For this reason, the future of implantable EP devices will simply eliminate the need for lead wires.
He showed a prototype Medtronic pacemaker the size of a Tylenol capsule with two tiny wire wings that act as both leads and a fixation device inside the heart. The device is implanted via catheter directly inside the heart with its arms anchoring it to the myocardium. For dual chamber pacing, two devices can be implanted and they will communicate with each other to synchronize the heart. The devices can be monitored using a smartphone.
Oesterle said this device was developed to replace the more conventional, but cumbersome, pacemaker and lead systems for use in developing countries. He said these complex systems need to be simplified so the average interventional cardiologist can implant them. As an example, he said India, one of the fastest growing cardiovascular device markets, has fewer than 80 electrophysiologists in the entire country. For this reason, the technology will allow pacing and resynchronization therapy to be brought to the masses.
Oesterle also said Medtronic is developing an even smaller pacemaker that is about one-third the length of a penny, which can be injected directly into the myocardium.
Nanostim is another company developing a catheter-deployed, leadless pacing device. St. Jude Medical has made a substantial investment in the company.
Permanent, Implantable Heart Failure Monitoring
A permanent wireless heart monitoring system for heart failure patients uses a new catheter implantable device the size of a paperclip and is powered by a wireless-based monitoring unit. The CardioMEMs system has been undergoing evaluation in the CHAMPION trial, with some patients having been implanted with the device for more than five years. Data so far shows a 28 percent reduction in heart failure hospitalizations.
St. Jude Medical’s left atrial pressure (LAP) management system is another permanent monitor for heart failure patients. Measurements are wirelessly transmitted to physicians, which can titrate drug doses to better manage their patients and prevent hospitalizations. It is currently under evaluation in the LAPTOP-HF (Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy) study, a pivotal investigational device exemption (IDE) trial.
Easier-to-Use, Less-Expensive FFR
While fractional flow reserve (FFR) is gaining favor thanks to guidelines to help determine whether a lesion should be stented, complaints persist that it adds more procedural time and requires an adenosine injection. However, a new technology called instantaneous wave-free ratio (iFR) can measure stenosis severity without adenosine and in less time.
Data presented from ADVISE, one of the late-breaking trials highlighted at TCT, showed the new measure yielded similar results to traditional FFR.
FFR is only used in 6 percent or less of percutaneous coronary intervention (PCI) cases in the United States. This is mainly due to its requiring adenosine, which minimizes and stabilizes coronary resistance during the test, but is uncomfortable for patients, and is time-consuming and expensive.
In ADVISE (Adenosine Vasodilation Independent Stenosis Evaluation), 157 stenoses (131 patients) were evaluated. Wave intensity analysis identified a period during the normal heart rhythm cycle where intracoronary resistance at rest is similar in variability and magnitude to those during FFR. The resting ratio of the distal-to-proximal pressure during this period, iFR, correlated closely with FFR.
“The clinical implications of the trial are that significant barriers to a physiological assessment of stenoses have been removed, and the new technique can potentially improve workflow in the cath lab, leading to a better patient experience and increased adoption,” said principal investigator Justin Davies, MBBS, MRCP, PhD. Dr. Davies is a clinical academic and interventional cardiologist at the National Heart and Lung Institute at Imperial College, London.
Volcano Corp., which was among the contributors to fund the study, has interest in commercializing the technology.
IVUS Computed Tomography
Volcano CEO Scott Huennekens said the company is developing a next-generation intravascular imaging system called Focused Acoustic Computed Tomography (FACT). The images produced by the sound-based system appear similar in resolution and image quality to optical coherence tomography (OCT) images.
The company also showed a prototype of a forward-looking intra-cardiac echo (FL-ICE) system for use in the growing number of structural heart cases now being treated in the cath lab and hybrid OR. Volcano is also working to commercialize devices combining IVUS with ablation, thrombectomy systems and CTO microcatheters.
Reducing TAVI Stroke Rates
While the PARTNER trail for the Sapien transcatheter aortic valve was very favorable and resulted in recent FDA clearance of the device, the trial also showed a higher rate of strokes in transcatheter aortic valve implantation (TAVI) patients over surgical patients. To reduce the risk of stroke, TAVI embolic protection devices are being developed.
SMT Research and Development is among the companies developing these devices. It showed its embolic protection device on the show floor at TCT. The filter consists of a mesh screen that is deployed on the aorta to cover the top of the aortic arch and prevent emboli from going into the left common carotid, brachiocephalic and subclavian arteries. The device is in trials in Europe.
Forward-Looking OCT for CTOs
California-based Avinger earlier this year gained CE mark for a forward-looking optical coherence tomography (OCT) system to aid recanalization of chronic total occlusions (CTOs). The company displayed the Ocelot system for the first time at TCT. It uses a 6 French catheter on a 110 mm shaft. The tip of the shaft has a screw tip, allowing operators to image where they want to go and to push or screw through the occlusion.
The company has already met with the FDA to discuss starting an IDE trial for the device in the U.S. by early 2012.





 April 25, 2023
April 25, 2023 









