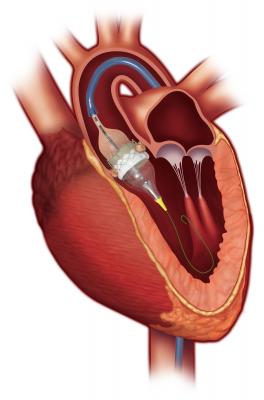
August 18, 2016 — The U.S. Food and Drug Administration (FDA) has expanded the indications for both the Edwards Lifesciences’ Sapien 3 and Sapien XT transcatheter heart valves for the treatment of patients suffering from severe, symptomatic aortic stenosis who have been determined by a heart team to be at intermediate risk for open-heart surgery. The Sapien valve is the first transcatheter aortic valve replacement (TAVR) therapy to obtain this indication in the United States.
“This is the first time in the U.S. that a transcatheter aortic valve has been approved for use in intermediate risk patients,” said Bram Zuckerman, M.D., director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health. “This new approval significantly expands the number of patients indicated for this less invasive procedure for aortic valve replacement.”
"The Sapien 3 valve has set a new standard for performance and patient outcomes with aortic valve replacement," said Vinod Thourani, M.D., co-director of Emory Heart and Vascular Center's Structural Heart and Valve Center and professor of cardiothoracic surgery at Emory University School of Medicine. "The clinical outcomes of 1.1 percent mortality and 1.0 percent disabling stroke at 30 days in this intermediate-risk population treated with the Sapien 3 valve are changing the paradigm of how we treat patients with aortic stenosis."
Thourani is the co-principal investigator of the Sapien 3 valve study.
Watch a video “TAVR Beats Surgery — Top News From ACC.16.” Dr. Thourani discusses the biggest news item from ACC.16 — the Sapien 3 TAVR device performed better that surgical aortic valve replacement.
"The intermediate-risk approval of the Sapien 3 valve is a major milestone, since it provides a less-invasive therapy that has demonstrated better outcomes for aortic valve patients, and is supported by the largest and most rigorous comparative body of evidence for the treatment of aortic stenosis," said Larry L. Wood, Edwards' corporate vice president, transcatheter heart valves.
The Sapien 3 valve intermediate-risk approval was based on data from a cohort of the PARTNER II Trial, which studied 2,005 intermediate-risk patients at 51 sites in the United States and Canada. The study demonstrated that patients treated with the Sapien 3 valve experienced clinically significant improvements for the composite primary endpoints of mortality, stroke, and moderate or severe aortic regurgitation at one year as compared to those treated with surgery. The data were presented in April at the American College of Cardiology (ACC) Annual Scientific Session and simultaneously published in The Lancet.
The expanded intermediate-risk indication granted by the FDA enables Heart Teams to treat patients with the Sapien 3 valve who they determine to have a predicted risk of surgical mortality of greater than or equal to 3 percent at 30 days, based on the Society of Thoracic Surgeons (STS) risk score and other clinical co-morbidities unmeasured by the STS risk calculator.
The Sapien 3 valve builds on Edwards' decades of experience in the development of tissue heart valves, and the proven benefits of the Edwards Sapien valves. The Sapien 3 valve was approved by the FDA in June 2015 for the treatment of patients with severe, symptomatic aortic stenosis who are at high-risk for open-heart surgery.
Medtronic announced Aug. 4 it received CE (Conformité Européenne) mark market clearance in Europe for its self-expanding, recapturable and repositionable CoreValve Evolut R TAVR system to patients at intermediate risk for open-heart surgery. The Evolut R System is the first transcatheter aortic valve replacement (TAVR or TAVI) device to obtain this expanded indication in Europe.
Watch the video “Declining TAVR Stroke Rates in Key Trials Presented at ACC.16.” Chandan Devireddy, M.D., assistant professor of medicine at Emory Healthcare in Atlanta and an investigator in the PARTNER trials, discusses the positive late-breaking PARTNER II and IIA data presented at ACC.16. Among the key measures in these trials was the stroke rate, which is now the same as surgery.
Changing the Standard of Care
Traditionally, open-heart surgery has been the gold standard for aortic valve replacement in intermediate risk patients, but it involves a larger incision and longer recovery time than the minimally invasive procedure used to insert the transcatheter heart valve. The FDA said about one-third of patients referred for open-heart surgery for aortic valve replacement fall into the “intermediate risk” category, which is defined as having a greater than three percent risk of dying within 30 days following surgery.
In a the PARTNER II clinical study to evaluate safety and effectiveness of the Sapien in intermediate patients, 1,011 aortic stenosis patients at intermediate risk for surgical complications were randomly selected to have a transcatheter aortic valve replacement procedure using the Sapien XT valve and 1,021 were randomly selected to have a traditional aortic valve replacement during open-heart surgery using a surgical tissue valve. In a second study, PARTNER IIa, 1,078 intermediate risk patients were implanted with the Sapien 3 valve; and outcomes in these patients were compared to the same group of 1,021 surgical control patients in the first study. The two studies demonstrated a reasonable assurance of safety and effectiveness of the Sapien XT and Sapien 3 devices in intermediate risk patients.
In January 2016, the FDA gacve approval for clinical trials testing the Corevalve and Sapien TAVR valves in low-risk surgical patients. Many experts believe if this trial shows outcomes similar to previous trials for high and intermediate risk patients, TAVR will become the standard of care. If this happens, it will eliminate the need for open heart surgery in most patients.
Read the article “Trends in Transcatheter Aortic Valve Replacement (TAVR)”
For more information: www.edwards.com


 November 14, 2025
November 14, 2025 









