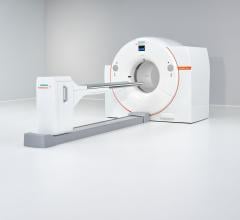
June 11, 2014 — GE Healthcare introduced its Discovery IQ PET/CT (positron emission tomography/computed tomography) system at the 2014 annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in St. Louis. The system enables both high image quality and intelligent quantitation. It is pending 510(k) clearance from the U.S. Food and Drug Administration (FDA) and not available for sale in the United States.
Discovery IQ
Physicians not only want the ability to detect smaller lesions, but also the ability to determine earlier whether the patient is responding to current treatment. With high sensitivity up to 22 cps/kBq, the largest axial field-of-view (up to 26 cm) and quantitative SUV measurements, Discovery IQ provides an essential tool to delivering personalized patient care. It enables physicians to see smaller lesions, scan faster with lower dose and improved image quality, read more efficiently, and reach more patients.
“By 2020, it’s estimated that 50 percent of people will develop cancer at some point in their lives, and we also know that currently approximately 70 percent of cancer patients do not respond to their initial chemotherapy treatment,” said Steve Gray, president and CEO of GE Healthcare MICT. “I’m excited to introduce the Discovery IQ to help physicians with their primary mission of delivering the best possible patient outcomes. And, we’ve focused on designing the system to make it as accessible as possible to more people in more places, to ensure high-performance PET/CT clinical care is available to whomever needs it.”
Some key features include:
- GE Healthcare’s Q.Clear PET reconstruction technology, providing up to two times improvement in both image quality and quantitation accuracy;
- A fully scalable system, allowing for the ability to upgrade as department needs change;
- Availability for the mobile market, enabling the latest technology across both fixed and mobile environments;
- New platform design and the new LightBurst detector technology, allowing for whole organ imaging via fast scans at low dose to help maximize patient comfort and safety; and
- Fast electronics and a dual acquisition channel that enables high quantitative accuracy for all clinically relevant tracers.
Q.Clear
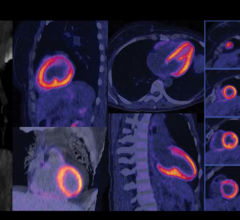
The Q.Clear technology is a critical component of Discovery IQ. It delivers, for the first time, no trade-off between image quality and quantitative SUV measurements. By providing two times improvement in both quantitative accuracy and image quality in PET/CT imaging, this innovative new tool provides benefits to physicians across the cancer care continuum from diagnosis, staging and treatment planning, to treatment assessment.
Over the last decade, PET image reconstruction technology has been designed to provide better image quality, reduced acquisition time and lower injected dose. Current PET iterative reconstruction technologies, such as Time of Flight (TOF) and OSEM, force a compromise between image quality and quantitation. The new Q.Clear technology shows the advantage of full convergence PET imaging with no compromise between quantitation and image quality.
CortexID Suite
CortexID software has been developed to aid physicians in the evaluation of patient pathologies via assessment and quantification of PET brain scans. The software aids in the assessment of human brain PET scans, enabling automated analysis through quantification of tracer uptake and comparison with the corresponding tracer uptake in normal subjects. The resulting quantification is presented using volumes of interest, voxel-based or 3-D stereotactic surface projection maps of the brain. The package allows the user to generate information regarding relative changes in PET-FDG glucose metabolism and in PET brain amyloid load between a subject’s images and a normal database, which may be the result of brain neurodegeneration.
For more information: www.gehealthcare.com

 June 23, 2025
June 23, 2025