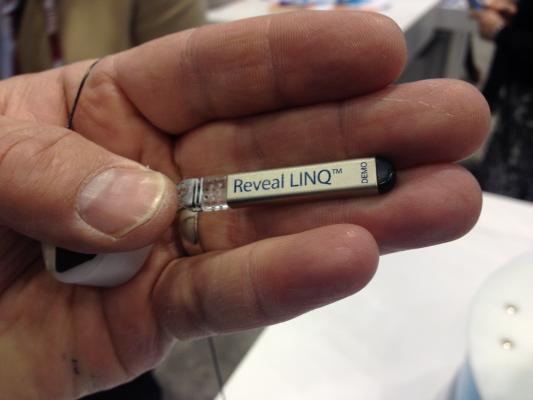
The Medtronic Reveal Linq implantable cardiac monitor is the smallest implantable monitor on the market. It is used as a long-term Holter monitor for 24-hour a day, 365-day a year patient monitoring. The device uses wireless connectivity to download the patient data with a patient bedside base unit to send the information over the internet so it is accessible by physicians.
May 16, 2017 - The study using small, subcutaneous implantable cardiac monitors for long-term, 24-hour a day monitoring, detected a high incidence of atrial fibrillation (AF) in patients previously undiagnosed but suspected to be at high-risk for AF and stroke. Data from the REVEAL AF (Incidence of AF in High Risk Patients) study were presented as a late-breaking session at Heart Rhythm 2017, the Heart Rhythm Society's 38th Annual Scientific Sessions.
The study found that at 18 months, continuous monitoring with either the Medtronic Reveal XT Implantable Cardiac Monitor (ICM) or the Reveal Linq ICM resulted in an AF detection rate of 29.3 percent among previously undiagnosed high-risk patients (based on clinical risk factors). The data showed continuous monitoring with an ICM detected AF beyond 18 months with a detection rate of 40 percent at 30 months. Additionally, 6.2 percent of patients were diagnosed with AF at 30 days, indicating that more than three-quarters of high risk patients with AF would have gone undetected with only 30 days of cardiac monitoring. The median time from device insertion to the first AF episode was 123 days, which is outside the range of conventional external monitoring.
The Reveal AF study also evaluated how physicians managed patients when AF was found. At least one clinical action was taken in 75.7 percent of patients who were diagnosed with AF (at 18 months). More than half (56.3 percent) of patients diagnosed with AF were prescribed oral anticoagulation by their physicians, which has been shown to significantly reduce stroke risk. This suggests that the information provided by the Reveal ICM was clinically meaningful.
"Detection of AF utilizing minimally invasive insertable cardiac monitors in a high-risk population combined with appropriate AF treatment could prevent many initial strokes," said James Reiffel, M.D., principal investigator of the REVEAL AF Study and professor emeritus of medicine, Department of Medicine, Division of Cardiology at the Columbia Presbyterian Medical Center in New York City. "Findings from the REVEAL AF study show that the rate of AF in patients at high-risk for AF and thus stroke but with no prior history of AF is significant, raising important public health implications on early screening and prevention of stroke in this demographic group."
AF is a common cardiac condition impacting millions worldwide in which the heart beats irregularly or rapidly.[1] Patients with AF are five times more likely to have a stroke [2] due to small blood clots that may form in the heart and subsequently travel to the brain. Failure to recognize and treat AF can lead to strokes, however, because AF often has no symptoms and may occur infrequently, it may not be detected by conventional cardiac monitoring techniques such as in-hospital monitoring, electrocardiography or traditional ambulatory cardiac monitors such as a Holter.[3-6] Unlike conventional monitoring methods, the Reveal Linq with TruRhythm Detection automatically and continuously detect and record abnormal heart rhythms for up to three years.
"AF is often undetected with conventional methods and only first diagnosed after the occurrence of a serious complication such as stroke which can result in significant impact to quality of life and even death," said Robert Kowal, M.D., Ph.D., vice president and medical director of the Cardiac Rhythm and Heart Failure division, which is part of the Cardiac and Vascular Group at Medtronic. "The REVEAL AF study provides important information to influence how physicians monitor high-risk patients to screen and treat AF, potentially preventing stroke from occurring."
REVEAL AF was a prospective, single-arm, multi-center study that sought to understand the incidence of adjudicated AF that lasted six minutes or more in high-risk patients who were previously undiagnosed with AF. The primary endpoint of the REVEAL AF study was AF detection rate at 18 months. A total of 385 patients received a Reveal XT ICM or a Reveal ICM and met the primary endpoint cohort definition. Patients were followed for a minimum of 18 months to monitor for AF, or up to a maximum of 30 months.
One-third the size of an AAA battery (~1 cc), the Reveal Linq ICM is inserted using a minimally invasive procedure and its presence is often nearly undetectable to the naked eye once the incision has healed. The device communicates wirelessly with a patient bedside monitor that uploads device data to the Medtronic CareLink network and is MR-Conditional, allowing patients to undergo magnetic resonance imaging (MRI), if needed. Earlier this year, Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Reveal Linq ICM with TruRhythm Detection which features improved accuracy to better identify abnormal heartbeats.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services of the highest quality that deliver clinical and economic value to healthcare consumers and providers around the world.
For more information: www.medtronic.com
References:
1. Chugh S, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014; 129:837-847.
2. Wolf PA, et al. Stroke. 1991; 22: 983-988.
3. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120-129.
4. Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011;124:477-486.
5. Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445-1452.
6. Ziegler PD, Glotzer TV, Daoud EG, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol.2012;110:1309-1314.


 December 19, 2025
December 19, 2025 









