March 14, 2017 — Climbing above 4,000 meters can provoke abnormal heart rhythms in otherwise healthy mountaineers, with the abnormalities increasing with altitude, new research has shown.
The study, by sports scientists at Leeds Beckett University (U.K.) and cardiologists at Poole Hospital, found that in a team of 16 healthy mountaineers without a previous history of heart disease, more than half (56.3 percent) experienced rhythm disturbances at altitudes of 4,100 meters or above.
These were either significant pauses in their heartbeat — where the heart stops for three seconds or more — or very fast or irregular heartbeats. The pauses generally took place at night, while the climbers were asleep. All identified abnormalities disappeared once climbers descended below 4,100 meters.
The research, published in the journal Circulation, was carried out with a team of military volunteers from the Defence Medical Services during an expedition to climb Nepal’s Mount Dhaulagiri, in the Himalayas.
The research is the first to continually monitor climbers’ heartbeat over an extended period and shows the potential for such technologies to help with medical diagnoses in remote locations.
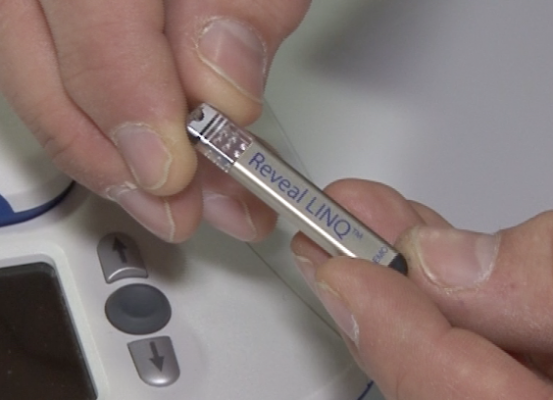
The group was studied over a period of at least four months, both before, during and after the 51-day expedition, using implantable cardiac monitors, called Reveal LINQ. Similar in size to a AAA battery the devices were developed by Medtronic. They are typically used in clinical care to detect rare cardiac events but had not previously been tried at high altitude.
Heart rate and activity data were continuously recorded by the device and could be uploaded to a remote stored server by a cellular signal or manually for analysis. The climbers could also trigger the device to specifically store extended recordings of their heartbeat if they experienced any concerning symptoms, such as severe palpitations, which was observed among half the participants. Additional data on the climbers’ blood oxygen levels and physical condition were also collected.
The most commonly identified cardiac abnormality were significant pauses, which were recorded on 81 occasions and affected 50 percent of the subjects. Both the frequency and duration of the pauses increased the higher the climbers went, with the longest pause lasting seven seconds. One climber experienced an episode of a sustained symptomatic irregular fast heartbeat known as atrial fibrillation that lasted for nearly five hours and had kept him awake at night, and another developed a fast abnormal heart rhythm know as a supraventricular tachycardia (SVT) lasting 31 seconds while he attempted to lift a heavy load.
Chris Boos, M.D., visiting professor at Leeds Beckett University and consultant cardiologist at Poole Hospital, who led this study explained: “Our research has delivered a unique and fascinating insight into cardiac physiology at high altitude, showing what happens to the heart rate and rhythm while you sleep and exercise at very high altitude.
“For the majority of mountaineers, there should be no cause for concern, as the pauses during sleep may simply represent normal physiological adaptation to the significant challenges of the very high altitude environment. However, going above 4,000 meters could potentially exacerbate any pre-existing conditions which climbers may or may not already be aware of, and this should be taken into account when planning expeditions.”
He added: “Our study also shows the potential of this kind of technology for delivering medical diagnoses to people in extremely remote locations.”
The team plans to continue its investigations with larger groups of subjects and using even more recent advances in cardiac non-invasive monitoring technology, which will enable even greater detail to be recorded without the need for devices to be implanted.
For more information: www.circ.ahajournals.org


 November 12, 2025
November 12, 2025 









