There have been several technology advances in PET/CT (positron emission tomography/computed tomography) systems recently introduced by vendors. On the show floors of the 2014 Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting and the 2014 Radiological Society of North America (RSNA) meeting, Philips, Toshiba and GE Healthcare introduced new PET/CT systems. You can view the PET/CT Systems comparison chart here.
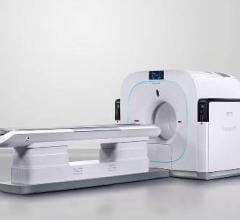
Philips Vereos First to Offer Digital Detectors
The Philips Vereos is the first PET/CT on the market to offer digital PET detectors, allowing improved image clarity over traditional analog photomultipliers. The system was first unveiled at RSNA 2013. It uses Philips’ digital photon counting technology in its digital silicon detectors. Philips said this results in a step change in performance that approximately doubles sensitivity gain, volumetric resolution and quantitative accuracy compared to analog systems. These radical improvements can ultimately be translated into high image quality, increased diagnostic confidence, improved treatment planning and faster workflows.
Toshiba’s Celesteion
Toshiba showed its first entry into the molecular imaging market when it unveiled its Celesteion PET/CT. The company hopes to attract buyers with what it describes as the industry’s largest bore, widest field-of-view, dose reduction technology and fast imaging. The bore is 90 cm for CT, 88 cm for PET. Field-of-view is 70 cm for both CT and PET, with an 85 cm CT extended FOV. Dose is minimized through Toshiba’s adaptive iterative dose reduction and Celesteion’s compliance with the Medical Imaging and Technology Alliance’s XR-29 Smart Dose Standard. The new PET/CT offers time-of-flight resolution and 0.5 sec CT rotation with 0.5 mm slices across a 32-slice detector.
Discover IQ
In addition, GE Healthcare introduced its Discovery IQ PET/CT system, which became U.S. Food and Drug Administration (FDA) cleared in September. It enables both high image quality and intelligent quantitation. GE said it delivers the highest NEMA (National Electrical Manufacturers Association) sensitivity (up to 22 cps/kBq) and the largest axial field-of-view (up to 26 cm) compared to other PET/CT equipment.
GE’s new Q.Clear image reconstruction software is a big component of Discovery IQ, eliminating trade-offs between image quality and quantitative SUV measurements. By providing two times improvement in both quantitative accuracy (SUV mean) and image quality (SNR) in PET/CT imaging, this new tool provides benefits to physicians across the cancer care continuum from diagnosis and staging to treatment planning and assessment. Over the last decade, PET image reconstruction technology has been designed to provide better image quality, reduced acquisition time and lower injected dose. Current PET iterative reconstruction technologies, such as time of flight (TOF) and ordered-subsets expectation maximization (OSEM), force a compromise between image quality and quantitation, GE said.
The Discovery IQ uses GE’s new LightBurst detector technology, allowing for whole organ imaging via fast scans at low dose. It also uses fast electronics and a dual acquisition channel that enables high quantitative accuracy for all clinically relevant tracers.
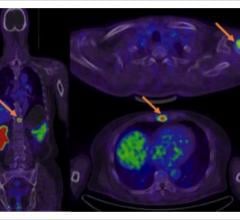
The Discover IQ includes the CortexID software, which aids physicians in the evaluation of patient pathologies via assessment and quantification of PET brain scans. It enables automated analysis through quantification of tracer uptake and comparison with the corresponding tracer uptake in normal subjects. The resulting quantification is presented using volumes of interest, voxel-based or 3-D stereotactic surface projection maps of the brain. The package allows the user to generate information regarding relative changes in PET-FDG glucose metabolism and in PET brain amyloid load between a subject’s images and a normal database, which may be the result of brain neurodegeneration.
Siemens Continuous Motion Bed
In 2013, Siemens Healthcare introduced the Biograph mCT Flow PET/CT system that overcomes the limitations of conventional bed-based PET/CT. It uses Siemens’ FlowMotion technology that moves the patient smoothly through the system’s gantry while continuously acquiring PET data. FlowMotion expands quantification in all dimensions for precise disease characterization in therapy monitoring, while enabling physicians to offer as low as reasonably achievable (ALARA) dose to every patient. The scanner uses a 78 cm bore with five-minute scanning and a continuous sense of progress throughout the scan provides the patient with a more comfortable exam experience.
Conventional PET/CT suffers from intrinsic sensitivity degradation from the center to the edge of the axial FOV. Overlapping sequential bed positions are used to compensate for this constraint, but this approach can lead to axially varying noise sensitivity. This noise can distort the quantitative values of a detected lesion, which may prompt a physician to mischaracterize the severity of a tumor. By continuously moving the patient through the detection system, FlowMotion technology eliminates overlapping bed acquisitions and maintains uniform noise sensitivity across the entire scan range.

 June 23, 2025
June 23, 2025