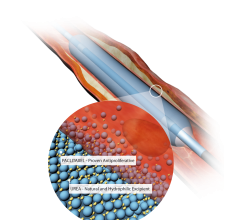
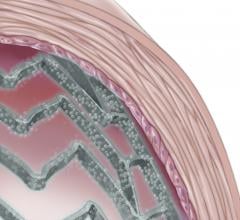
June 16, 2014 — C. R. Bard Inc. announced that the U.S. Food and Drug Administration’s (FDA) Circulatory System Devices ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
June 3, 2014 — Medtronic Inc. announced CE mark and launch of the NC Euphora noncompliant balloon dilatation catheter ...
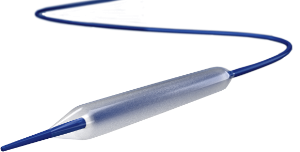
May 23, 2014 — The first drug-eluting balloon to go up for review by the U.S. Food and Drug Administration (FDA) will be ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 14, 2014 — OrbusNeich announced the launch of the Sapphire II NC coronary dilatation catheter, a non-compliant ballo ...
April 23, 2014 — Interim results from The Chocolate Bar Registry conducted in the United States demonstrate that use of ...
April 16, 2014 — Patients with peripheral artery disease in the upper leg experienced significantly better outcomes at ...
March 6, 2014 — Despite good immediate results, in up to 40 percent of patients, obstructed arteries in the leg treated ...
March 3, 2014 — Biotronik announced the release of its Passeo-18 Lux drug-releasing balloon (DRB) in all countries ...
February 28, 2014 — Cordis Corp. announced an agreement with TriReme Medical Inc. that grants the company exclusive ...

Herbert Aronow, M.D., MPH, St. Joseph Mercy Hospital, Ann Arbor, and an active member of ACC and SCAI, explains the top ...
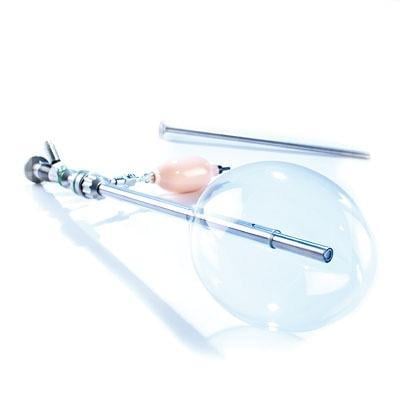


 June 16, 2014
June 16, 2014