June 10, 2009 – CardioDynamics yesterday said it entered into a definitive merger agreement whereby SonoSite will ...
Cardiac Diagnostics
This channel includes news, videos, podcasts and other content on new technology innovations for cardiac diagnostic systems and techniques. This includes laboratory testing, blood tests including troponin testing, electrocardiogram (ECG) systems, point of care testing systems, genetic testing, cardiac patient monitoring devices including wearable sensors, and studies showing new ways to diagnose heart diseases.
June 5, 2009 - Utilizing next generation sequencing and the latest advancements in sequence capture, genomic and ...
June 4, 2009 - LifeSync Corp. this week said Florida Hospital in Orlando completed implementation of the LifeSync ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
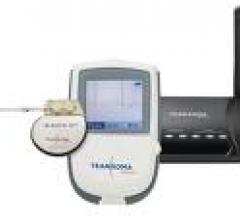
June 2, 2009 - Transoma Medical Inc., manufacturer of Sleuth and Sleuth AT wireless, automated implantable cardiac ...
June 2, 2009 - IDEAL LIFE Inc. and home care agency Bayada Nurses Inc. said today hospitalizations for patients ...
May 29, 2009 - Saint Francis Hospital in Evanston, Ill. has become the first hospital in Illinois to offer the ...
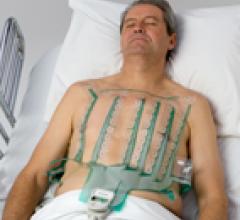
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 26, 2009 – Biotronik Inc. recently launched its Lumax 540 series implantable cardioverter defibrillators ...
May 18, 2009 - CardioNet Inc. launched SomNet, a new clinical indicator for severe sleep disorders available in ...
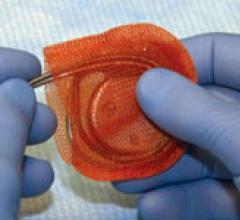
May 18. 2009 - TYRX Inc. announced the initial, interim clinical results from its AIGISRX Antibacterial Envelope ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
May 18, 2009 – At Heart Rhythm 2009 last week Biotronik said it completed the first enrollment in the pivotal ...
May 14, 2009 - St. Jude Medical Inc. yesterday announced European CE mark approval of its Accent RF pacemaker and ...
May 13, 2009 - Medtronic Inc. today announced the start of a nationwide trial to examine the relationship between ...
Sudden cardiac death (SCD) is the leading medical cause of death in young athletes and its impact is consistent worldwide. Most professional athletes in the United States are required to take part in comprehensive cardiovascular screening programs to identify often-asymptomatic congenital or inherited heart disorders, and other cardiac risk factors. There remains a debate however, whether to mandate ECGs as part of pre-participation screening programs for student athletes at the collegiate and high school levels or even at younger ages.
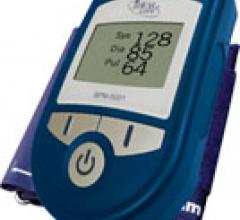
May 11, 2009 - With 12 million Americans at risk for sudden cardiac arrest (SCA), Cambridge Heart’s Microvolt T ...
May 10, 2009 - Biosite and the FDA notified healthcare professionals of the Class 1 recall of the Biosite brand ...
May 7, 2009 - The University of Illinois Medical Center, the primary teaching facility for the UIC College of ...

 June 10, 2009
June 10, 2009