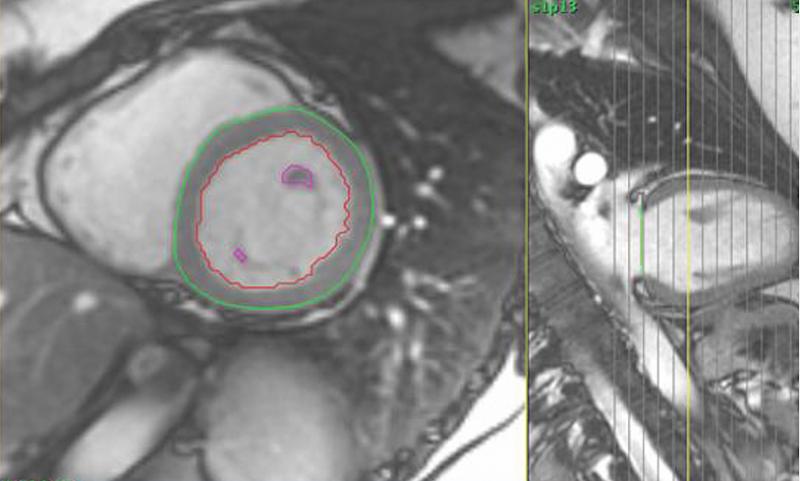
October 29, 2020 — Contrast agents used to improve views of the heart on magnetic resonance imaging (MRI) carry a very ...
Contrast Media
Contrast media, also called contrast agents, are used to enhance the blood and perfusion in tissues.This includes iodine based contrast for on computed tomography (CT), gadolinium based agents for MRI and lipid bubble contrast agents used in ultrasound.
October 4, 2019 – RenalGuard Therapy was found to be superior to the POSEIDON method in preventing contrast-induced ...
August 28, 2019 — A new 3-D magnetic resonance imaging (MRI) computing technique developed by scientists in WMG at the ...
August 7, 2019 — Heart failure is the fourth most common cause for all admission to U.S. hospitals, and it is the most ...
July 15, 2019 — The U.S. Food and Drug Administration (FDA) has approved Gadavist injection for use in cardiac magnetic ...
Sharon Mulvagh, M.D., FASE, FACC, FRCPC, professor of medicine, division of cardiology, Dalhousie University, Halifax ...
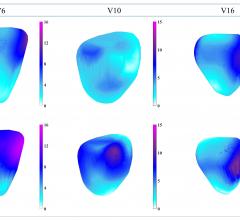
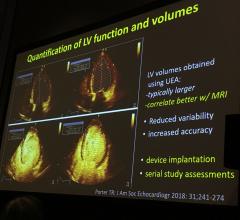
Thomas Porter, M.D., FASE, the Theordore F. Hubbard Distinguished Chair of Cardiology and a professor of medicine at the ...
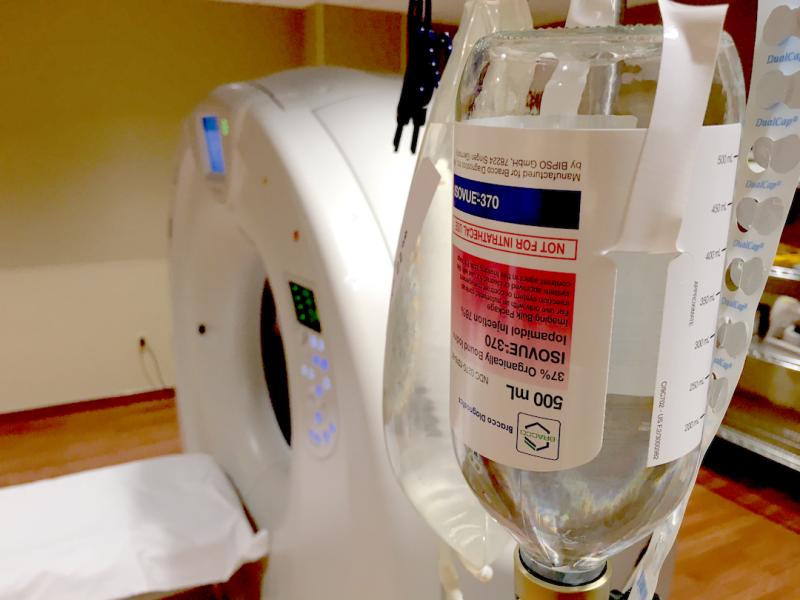
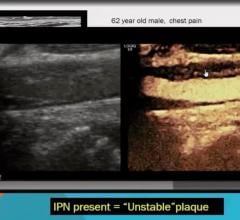
Iodine-based contrast agents used in computed tomography (CT) and catheter-based angiography have been implicated as a ...
As part of the Consolidated Appropriations Act of 2018, pass-through payment status for LUMASON® (sulfur hexafluoride ...
May 9, 2019 — Osprey Medical announced the launch of DyeMINISH, a global patient registry to evaluate the ongoing safety ...
March 7, 2019 — National and international ultrasound societies are urging the U.S. Food and Drug Administration to ...
November 30, 2018 — VigiLanz and Cincinnati Children’s Hospital Medical Center recently announced a collaboration that ...
September 21, 2018 — Osprey Medical announced a collaboration with GE Healthcare on Osprey’s Be Kind to Kidneys campaign ...
In February 2018, a workshop was held at the National Institutes of Health (NIH) in Bethesda, Maryland, to explore ...
Thomas Porter, M.D., professor of medicine and director of the echocardiography lab at the University of Nebraska ...

 October 29, 2020
October 29, 2020