Payam Dehghani, M.D., FRCPC, FACC, FSCAI, co-director of Prairie Vascular Research and associate professor at the ...
Coronavirus (COVID-19)
This page contains medical information for clinicians on the 2019 Novel Coronavirus (COVID-19, also called 2019-nCoV, and now clinically SARS‐CoV‐2). This section includes articles that pertain to clinicians and cardiologists on the virus, new technologies being deployed to fight the virus and clinical information from various sources. Here are direct links for medical professionals to COVID-19 resources from the U.S. Food and Drug Administration (FDA), Centers for Disease Control (CDC) and the World Health Organization (WHO). Daily world-wide statistics on the coronavirus outbreak are available from the WHO Situations Reports. Here is the Centers for Medicare and Medicaid Services (CMS) frequently asked questions and answers (FAQs) for healthcare providers regarding Medicare payment for laboratory tests and other services related to the COVID-19.
May 4, 2021 – A new study, presented today at the 2021 American Association for Thoracic Surgery (AATS) 101st annual ...

May 3, 2021 – One third of patients will die who have COVID-19 (SARS-CoV-2) and suffer a ST-elevated myocardial ...
During the COVID-19 pandemic, many cardiology departments were faced with the daunting task of supporting inpatient and ...
April 28, 2021 – Results from a retrospective observational study, presented today at Society for Cardiovascular ...
April 20, 2021 – The Minneapolis Heart Institute Foundation (MHIF) announced the first publication of outcomes from the ...
(This story was updated May 7, 2021 in the last subheaded section)
April 13, 2021 — The U.S. Food and Drug ...
COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...
April 6, 2021 — A tsunami of chronic health conditions as a result of the COVID-19 (SARS-CoV-2) pandemic, especially car ...
Behnood Bikdeli M.D., a cardiologist at the Brigham and Women’s Hospital, Harvard Medical School, Boston, offers an ...
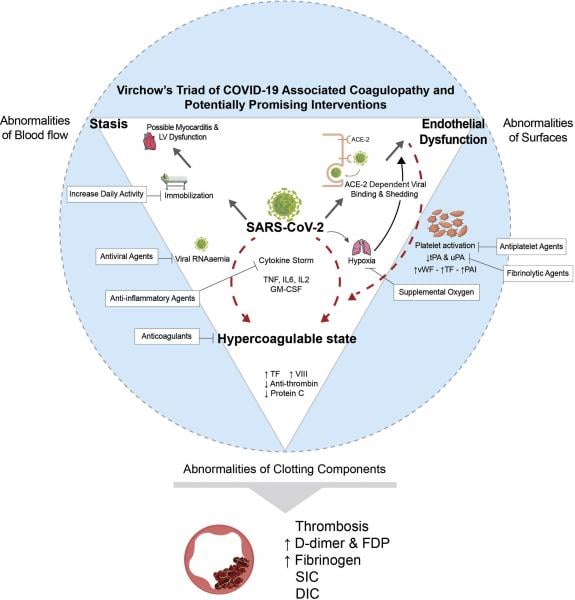
A comprehensive review or more than 80 randomized controlled trials (RCTs) investigating how to best manage optimal ...
March 17, 2021 — Around 50% of patients who have been hospitalized with severe COVID-19 (SARS-CoV-2) and have damage to ...

March 12, 2021 — Since early in the pandemic, COVID-19 has been associated with heart problems, including reduced ...
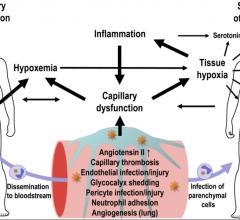
March 12, 2021 — A new review suggests that blood vessel damage and impaired oxygen delivery related to COVID-19 play a ...

The latest cardiology practice-changing scientific breakthrough, late-breaking study presentations have been announced ...
March 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
March 1, 2021 — The U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the third ...


 May 06, 2021
May 06, 2021