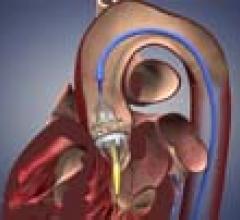
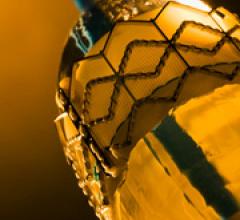November 18, 2011 – Boston Scientific’s Watchman left atrial appendage (LAA) closure device has been implanted in the ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
November 18, 2011 – W. L. Gore & Associates Inc. announced that it has received approval from the U.S. Food and Drug ...
November 18, 2011 —The Berlin Heart Group announced today that clinical trial data were presented this morning at the ...
November 18, 2011 – Medtronic and Bain Capital, a global private investment firm, today announced they have entered into ...
Each year that I attend Transcatheter Cardiovascular Therapeutics (TCT), I comb the event and its sessions looking for ...
November 16, 2011 — Heart failure patients with a previous myocardial infarction showed an average of 12 percent ...
November 16, 2011 – Johnson & Johnson Pharmaceutical Research & Development announced that adding oral rivaroxaban to ...
November 15, 2011 — A two-year study of patients in the landmark PARTNER trial confirms the one-year findings and ...
November 15, 2011 – Results of the PARTNER Cohort A QOL study demonstrate that transcatheter aortic valve replacement ...
November 14, 2011 — Edwards Lifesciences Corp. announced data were presented on the quality of life impact and cost ...
November 11, 2011 — Maquet Cardiovascular announced U.S. Food and Drug Administration (FDA) 510(k) clearance and CE mark ...
November 10, 2011 — The results of an analysis of 149 EVEREST II high surgical risk patients with significant mitral ...
November 10, 2011 — Edwards Lifesciences Corp. announced the U.S. Food and Drug Administration (FDA) conditionally ...
November 9, 2011 – Abiomed, a provider of heart support technologies, announced results of an economic study at the ...
November 8, 2011 – Medtronic today announced it received CE (Conformité Européenne) mark for the CoreValve System to be ...

 November 18, 2011
November 18, 2011