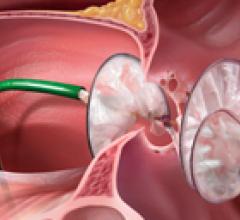
The Society for Cardiovascular Angiography and Interventions (SCAI) has partnered with the American College of ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
March 18, 2008 - Abiomed Inc. received conditional approval from the FDA to begin its Impella 2.5 Circulatory Support ...
March 11, 2008 – The FDA cleared an investigational device exemption (IDE) application from Life Recovery Systems to use ...
March 14, 2008 - Thirteen of the 18 top-ranked U.S. News and World Report hospitals (72.2 percent) use the noninvasive ...

Choosing the right device to regulate or jump-start the heart can be a challenge. Not only does a patient’s ...
March 7, 2008 – CircuLite has advanced its Synergy Pocket Circulatory Assist Device clinical program into a 20 ...
March 5, 2008 - Edwards Lifesciences Corp. reported that the first three human implants of the next-generation ...
February 27, 2008 - CircuLite Inc. released preliminary results of a pilot first-in-man study of four patients who ...
February 27, 2008 - The Center for Connected Health, a division of Partners HealthCare and EMC Corp. will study how EMC ...

There are several patient warming and cooling devices available for temperature management, but the practice of induced ...
W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder ...
February 11, 2008 – A new minimally invasive vascular occlusion device designed to close a patent ductus ...
February 11, 2008 - Leman Cardiovascular’s animal studies to evaluate the performance in both the aortic and mitral ...
CryoLife Inc.'s CryoValve SG pulmonary human heart valve is processed with the company’s proprietary SynerGraft ...
February 8, 2008 - CryoLife Inc. received 510(k) clearance from the FDA for its CryoValve SG pulmonary human heart ...

 March 16, 2008
March 16, 2008