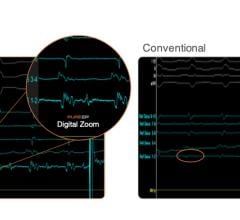
Sept. 2, 2025 — Stereotaxis has announced the successful completion of the world’s first procedures using MAGiC Sweep, a robotically-navigated high-density electrophysiology (EP) mapping catheter. The ...
EP Mapping and Imaging Systems
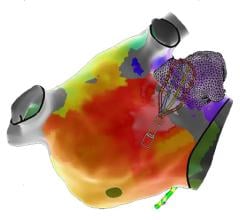
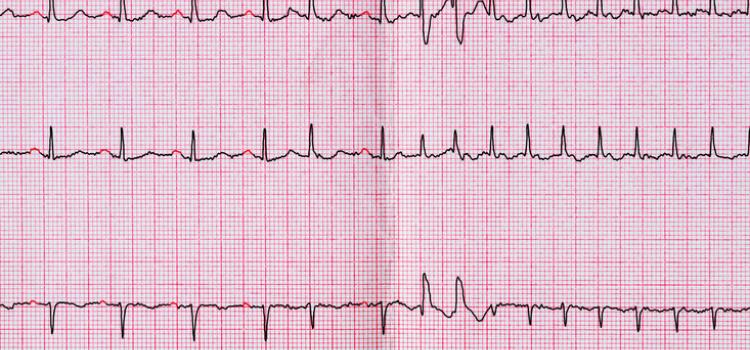
This channel page contains news on new technology innovations for electrophysiology (EP) mapping and imaging systems used to guide transcatheter cardiac ablation procedures. These systems use mapping catheters that contain electrodes that measure the electrical activity of the cardiac tissue. This is transferred into mapping system software where a 3D model is created of the heart, a color-coded overlay showing the electrical waves generated during each heart beat, the touch points where the tissue was mapped, and showing the location of the catheter inside the heart. Tissue identified as having unhealthy electrical activity that is cause an arrhythmia can then be ablated directly or isolated using an ablation catheter to cause small burns/scar tissue that block electrical signals.
Sept. 2, 2025 — Stereotaxis has announced the successful completion of the world’s first procedures using MAGiC Sweep, a ...
Sept. 3, 2025 — Kardium Inc. recently announced it has received pre-market approval (PMA) for the Globe Pulsed Field ...
July 19, 2023 — Stereotaxis, a pioneer and global leader in surgical robotics for minimally invasive endovascular ...
May 24, 2023 — Stereotaxis announced a global collaboration with Abbott to integrate Abbott’s EnSite X EP System with ...
May 17, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmiatreatment and part of Johnson & Johnson ...
May 15, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson ...
May 2, 2023 — The Heart Rhythm Society (HRS) is making final preparations for its Annual Heart Rhythm Meeting, HRS2023 ...
December 14, 2022 — Field Medical, Inc. and CardioNXT, Inc. announce a strategic collaboration to provide the first-of ...
May 2, 2022 – Johnson & Johnson MedTech have announced that Biosense Webster, Inc., a worldwide leader in the science ...
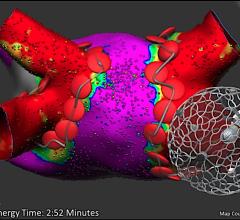
April 29, 2022 – Vektor Medical has announced positive results from its “Vektor vMap Clinical Validation Study” ...
April 27, 2022 – CathVision, a medical technology company developing innovative electrophysiology solutions designed to ...
March 3, 2022 – CIRCA Scientific, Inc. has announced that its S-CATH M Esophageal Temperature Probe is now cleared for ...
January 14, 2022 — Medtronic has entered into a definitive agreement to acquire Affera Inc., a Boston area-based ...
January 13, 2022 — Abbott received U.S. Food and Drug Administration (FDA) clearance for the EnSite X EP System with ...
November 22, 2021 — BioSig Technologies Inc., a medical technology company commercializing an innovative biomedical ...




 September 05, 2025
September 05, 2025