May 22, 2014 — A study presented at Heart Rhythm 2014, the Heart Rhythm Society’s 35th Annual Scientific Sessions ...
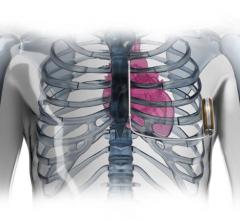
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).

May 19, 2014 — St. Jude Medical announced that data presented during a late-breaking clinical trial session at the 2014 ...

May 13, 2014 — The Heart Rhythm Society (HRS), American College of Cardiology (ACC) and American Heart Association (AHA) ...

May 13, 2014 — A Boston Scientific Corp. study demonstrated that outcomes for patients with the company's transvenous im ...
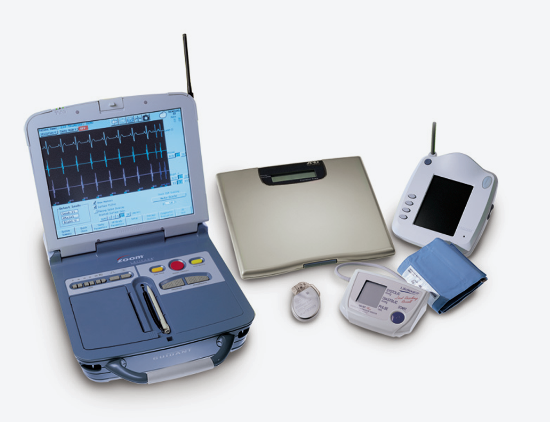
May 12, 2014 — Patients using the Boston Scientific Latitude remote patient management system with wireless telemetry ...
May 6, 2014 — Medtronic announced the first U.S. implant of the Evera MRI SureScan implantable cardioverter ...
April 28, 2014 — Boston Scientific Corp. has conducted the first implant in the clinical trial of the next-generation ...
April 24, 2014 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for its latest ...
April 23, 2014 — Three weeks after delivering her first child, Amanda began to suffer from extreme fatigue, headaches, a ...
April 16, 2014 — Biotronik announced that the first patients across the United States have received implants of the new ...

April 11, 2014 — Medtronic announced CE mark and European launch of the Evera MRI SureScan implantable cardioverter ...
April 11, 2014 — Boston Scientific Corp. has expanded the launch of its S-ICD (subcutaneous implantable cardioverter ...
April 10, 2014 — Cardiologists at Arnold Palmer Hospital for Children performed Florida’s first pediatric implant of a ...

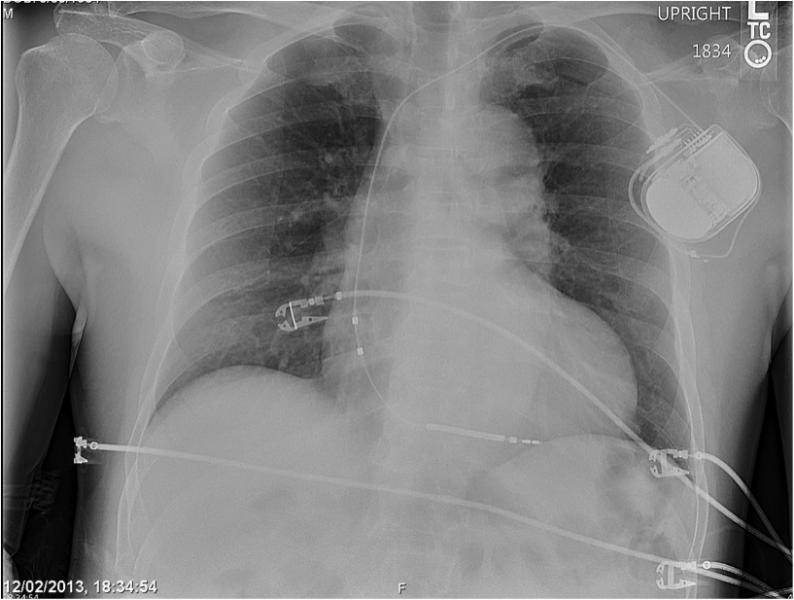


 May 22, 2014
May 22, 2014