This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
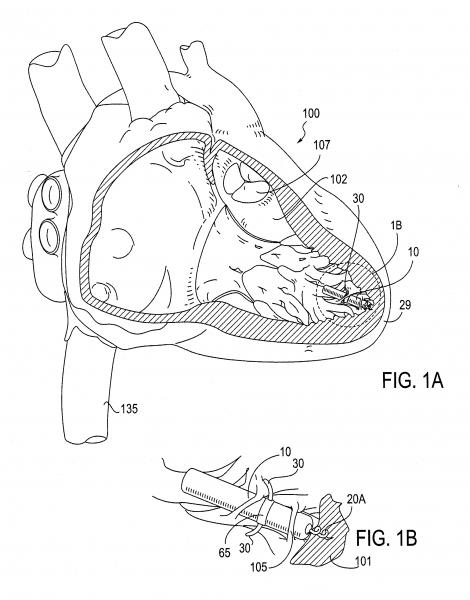
June 26, 2013 — Validating its shock reduction research and technology, Medtronic Inc. announced the results of three cl ...
May 20, 2013 — A device commonly used to treat dangerous heart rhythms may cause more issues for patients than a simpler ...
May 16, 2013 — The incremental mortality in implantable pacemaker and defibrillator recipients who experience a device ...
May 16, 2013 — New data from 100,438 patients with Boston Scientific implantable cardioverter defibrillators (ICDs) and ...
May 16, 2013 — St. Jude Medical Inc. has received CE Mark approval of its Ellipse and SJM Assura portfolio of implantabl ...
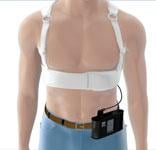
May 14, 2013 – Wearable cardioverter defibrillators (WCD) can be an effective therapy option for patients with a ...
May 14, 2013 — Biotronik announced the U.S. Food and Drug Administration (FDA) granted approval for its Ilesto 7 implant ...
May 13, 2013 — The Population Health Research Institute (PHRI) has conducted a further independent analysis of data ...
April 8, 2013 — Tyrx Inc. has received a license from Health Canada to market its new AigisRx R Fully Resorbable ...

March 27, 2013 — Biotronik received CE mark for its most advanced ICD/CRT-D (implantable cardioverter defibrillator/card ...

March 21, 2013 — The American College of Cardiology (ACC) and Heart Rhythm Society (HRS), along with key specialty ...

March 11, 2013 — Even minor weight loss is associated with worse health outcomes among patients implanted with a certain ...
Oklahoma Heart Hospital (OHH), Oklahoma’s first dedicated heart hospital, has excelled at creating quality outcomes ...