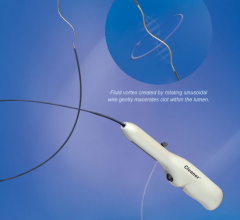
January 22, 2013 — Using tiny balloons to temporarily block blood flow to the uterus during a high-risk Caesarean ...
Interventional Radiology
Interventional radiology uses tools like embolization devices to provide minimally invasive medical diagnosis and treatment using images.

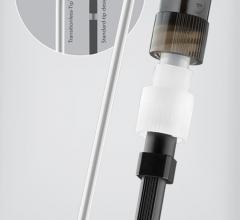
January 16, 2013 — Rex Medical L.P. announced that it has received 510(k) Clearance from the U.S. Food and Drug ...
Mercy Hospital in Chicago developed an interventional program around its hybrid cath labs, fostering collaboration ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Mercy Hospital in Chicago has developed a successful hybrid cath lab program where various specialties work together for ...
October 15, 2012 — Surefire Medical Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market ...
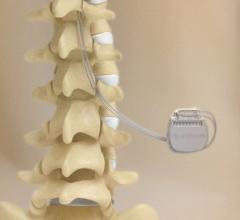
August 30, 2012 — Spinal cord stimulation (SCS) improves heart function and could become a novel treatment option for ...

Interventional procedures are growing rapidly and becoming more specialized in diverse areas of care. In the next ...
July 27, 2012 — Cook Medical announced general availability of the Aprima Access nonvascular introducer set, the first ...
This video, provided by Crux Biomedical, demonstrates the implantation of the FDA-cleared Crux VCF inferior vena cava ...

Cath lab volumes have gone down in recent years for several reasons, but one of the biggest reasons might be market ...
June 21, 2012 — Surefire Medical Inc. announced that it has received 510(k) U.S. Food and Drug Administration (FDA) ...
June 18, 2012 — St. Jude Medical Inc. announced it has received U.S. Food and Drug Administration (FDA) clearance and ...
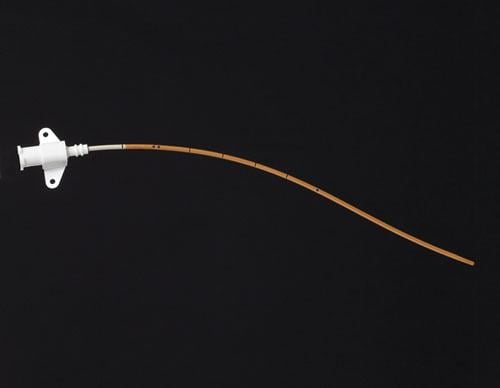
Peripherally inserted central catheters (PICCs) are devices used for intravenous access to facilitate the delivery of ...
May 30, 2012 — Boston Scientific Corp. announces the U.S. Food and Drug Administration has cleared an expanded use ...
May 1, 2012 — Theragenics Corp. announced that Galt Medical, a wholly-owned subsidiary of Theragenics, has received U.S ...

 January 22, 2013
January 22, 2013