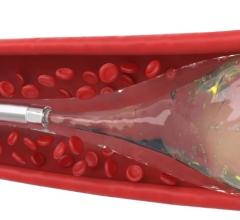
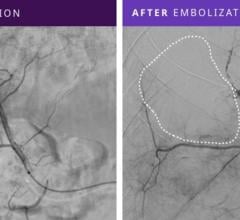
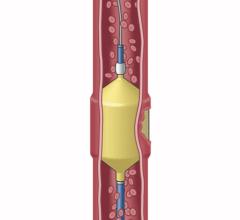
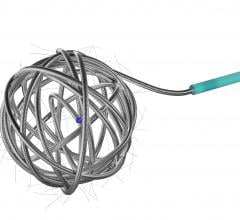
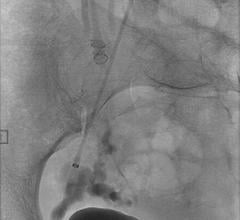
June 18, 2012 — St. Jude Medical Inc. announced it has received U.S. Food and Drug Administration (FDA) clearance and will immediately launch the Amplatzer Vascular Plug 4 (AVP 4) for use in transcatheter embolization procedures (minimally invasive procedures that involve the selective blocking of blood vessels) within the peripheral vasculature. Approximately 50,000 peripheral embolizations are performed each year in the United States.
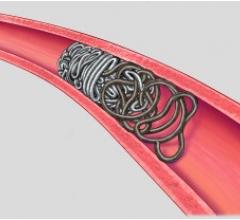
As the industry’s first vascular plug that can be delivered using a standard diagnostic catheter, the AVP 4 offers physicians the ability to treat smaller and more difficult-to-access blood vessels using vascular plug technology. The AVP 4 also provides full cross-sectional vessel coverage and may be recaptured and repositioned prior to deployment, allowing physicians to block a vessel with greater precision and speed than is achievable with conventional embolic coils.
Vascular plugs assist physicians in quickly blocking or rerouting blood flow by occluding (closing) abnormal blood vessels. Vascular occlusion lessens pressure on malformed, weakened or leaking blood vessels and reduces blood supply to benign or malignant tumors. The current standard of care for vascular occlusion involves placing several individually threaded coils through a catheter and packing them together to block blood flow.
The Amplatzer Vascular Plug 4 received CE mark approval in July 2009.
For more information: www.sjmavp4.com


 June 05, 2025
June 05, 2025