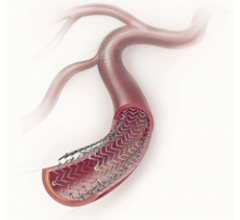
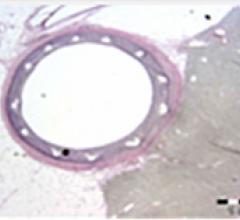
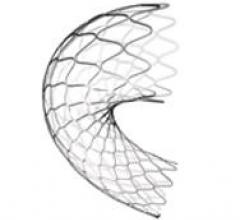
The Holy Grail sought for more than two decades by interventional cardiology has been proof that minimally invasive ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
October 7, 2011 — Biosensors has announced completion of its acquisition of JW Medical Systems Ltd. (JWMS), one of the ...

October 4, 2011 – Abbott today said it began enrolling U.S. patients in the EXCEL (Evaluation of Xience Prime or Xience ...

There have been several key news items from the U.S. stent market over the past year. These include the introduction of ...
September 13, 2011 – An U.S. Food and Drug Administration’s (FDA) will discuss recommendations for Cook Medical Zilver ...
August 31, 2011 — Results of a recent randomized controlled study show the drug-eluting stent Xience V performs well in ...
August 17, 2011 — OrbusNeich announced its Genous stent showed no significant difference in target vessel failure (TVF) ...
August 9, 2011 — Boston Scientific Corp. said the U.S. Court of Appeals for the First Circuit has affirmed the dismissal ...
July 29, 2011 — Boston Scientific Corporation's board of directors has approved a five-year, $150 million investment to ...
July 27, 2011 – Micell Technologies Inc. announced that it has completed patient enrollment in its DESSOLVE II CE mark ...
July 26, 2011 – The Resolute drug-eluting stent (DES) from Medtronic Inc. showed superiority to Boston Scientific Corp ...
April 26, 2011 – The U.S. Food and Drug Administration (FDA) announced this week it cleared Boston Scientific’s Ion ...
The goal of treating peripheral artery disease (PAD) in the superficial femoral artery (SFA) is limb salvage and ...

Navigating through the world of coronary stents is about to become more complex. A plethora of new products are on the ...
June 30, 2011 — Micell Technologies Inc. announced that it has completed its review of the scheduled four-month follow ...


 October 11, 2011
October 11, 2011