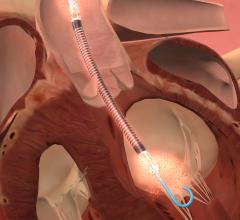
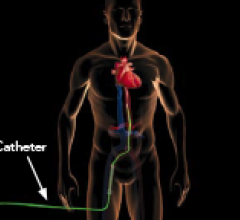
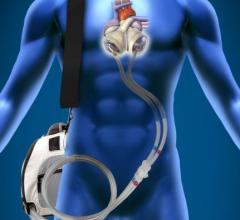
April 7, 2015 — An expert consensus statement released by four leading cardiovascular societies provides new guidance on ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
April 2, 2015 — SynCardia Systems Inc. has received U.S. Food and Drug Administration (FDA) approval to conduct an ...
March 24, 2015 – The U.S. Food and Drug Administration (FDA) has granted pre-market approval (PMA) clearance for Abiomed ...
January 30, 2015 — SynCardia Systems Inc. has received U.S. Food and Drug Administration (FDA) approval to conduct a cli ...
January 27, 2015 — NewYork-Presbyterian Hospital/Columbia University Medical Center is one of a select group of medical ...
October 9, 2014 — CardiacAssist Inc. announced that the first Protek Duo veno-venous extracorporeal life support (VV ...
September 16, 2014 – Clinical trial results by Recover Right revealed a survival rate of 73 percent in the total patient ...
October 6, 2014 — St. Vincent Heart Center officials announced that the faith-based hospital is the second in the nation ...
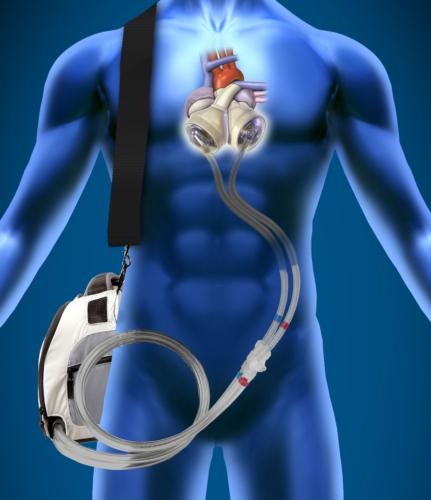
September 22, 2014 — Robert Littlefield, MSc, GlobalData's senior analyst covering Medical Devices, said:
“Since the ...
September 5, 2014 — CardiacAssist announced it has received 510(k) clearance from the U.S. Food and Drug Administration ...
August 29, 2014 — CardiacAssist announced it has received a Class 2 medical device license from Health Canada for its ...
August 13, 2014 — Celladon Corp. announced that the first patient has been dosed in a clinical trial titled ...
August 11, 2014 — SynCardia Systems Inc. received approval in July from the U.S. Federal Drug Administration (FDA) for ...
August 7, 2014 — The Berlin Heart Group announced they have completed enrollment in their post-approval study, the only ...

 April 07, 2015
April 07, 2015