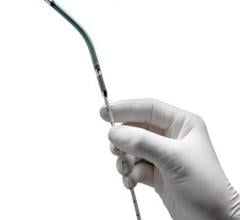
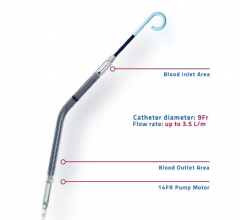
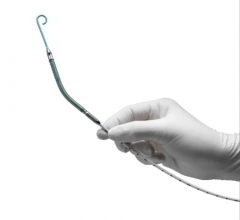
March 8, 2017 — Congestive heart failure patients at the University of Alabama at Birmingham (UAB) now have reason for ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
February 24, 2017 — Abiomed Inc. announced that it has supported more than 50,000 patients in the U.S. with its Impella ...
January 31, 2017 — To save the life of an 11-year-old boy, Ann & Robert H. Lurie Children’s Hospital of Chicago has ...
January 17, 2017 — Investigators at the University of Utah have identified distinct differences in the hearts of ...
January 3, 2017 — The Impella CP heart pump (Abiomed) demonstrated no improvement in mortality for patients with ...

The capsule endoscopy systems offer a hands-free and highly accurate imaging modality for healthcare practitioners. A ...
December 8, 2016 — St. Jude Medical Inc. announced results of the MOMENTUM 3 U.S. IDE Clinical Study during a late ...
December 7, 2016 — Abiomed Inc. announced it has expanded its U.S. Food and Drug Administration (FDA) pre-market ...
October 27, 2016 — Abiomed Inc. said the U.S. Food and Drug Administration (FDA)gave approval for a prospective ...
October 26, 2016 — The U.S. Food and Drug Administration (FDA) provided an update and additional information regarding ...
October 5, 2016 — Medtronic said two previously communicated global voluntary recalls related to the HeartWare ...

New cardiovascular device therapies for atrial fibrillation (AF) and heart failure (HF) are rapidly evolving with the ...
August 29, 2016 — SynCardia Systems, manufacturer of the Total Artificial Heart (TAH), announced that the judge ...
September 9, 2016 — Carmat announced that the first implantation of its bioprosthetic artificial heart within the ...
August 11, 2016 — Carew Medical Wear Inc., a custom medical outfit company specializing in left ventricular assistance ...


 March 08, 2017
March 08, 2017