August 1, 2014 — For the first time, cardiac surgeons, medical professionals and the public can watch the implantation ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
July 18, 2014 — The Freedom portable driver has received U.S. Food and Drug Administration (FDA) approval for use with ...
Mr. KH is a 58-year-old male with a past medical history of hypertension and tobacco abuse who presented to the ...
July 7, 2014 — Thoratec Corp. has acquired Apica Cardiovascular Ltd. for an upfront cash payment of $35 million and ...

Interventional thought leaders at the American College of Cardiology (ACC) 2014 meeting shared their predictions about ...
May 7, 2014 — HeartWare Intl. Inc. issued a voluntary Urgent Medical Device Correction related to all HeartWare ...
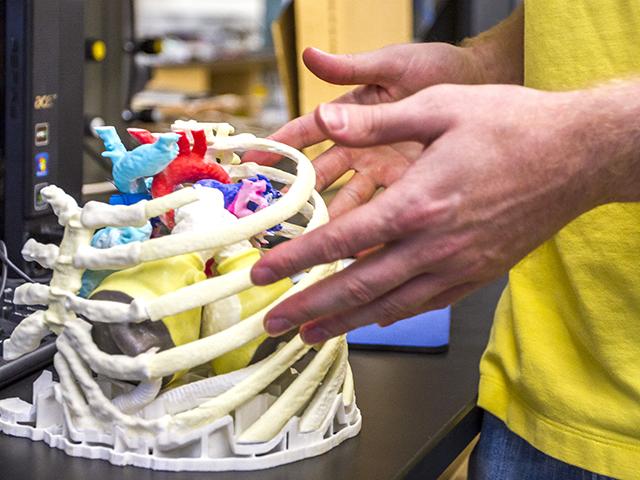
April 28, 2014 — An Arizona State University research group and medical professionals at Phoenix Children’s Hospital are ...

April 25, 2014 — Vanderbilt Heart and Vascular Institute (VHVI) was the first facility in Tennessee to implant the ...
April 23, 2014 — Three weeks after delivering her first child, Amanda began to suffer from extreme fatigue, headaches, a ...
April 18, 2014 — New research presented at the 34th annual meeting of the International Society for Heart and Lung ...
April 14, 2014 — CardiacAssist announced it has received a Class 4 medical device license for its TandemHeart system ...
April 10, 2014 — The largest single-center study of patients implanted with a total artificial heart found the device ...

Percutaneous ventricular assist (pVAD) devices offer more hemodynamic support than the 40-year-old gold standard of intr ...
March 18, 2014 — Thoratec Corp. initiated a voluntary worldwide Medical Device Correction in order to update its ...



 August 01, 2014
August 01, 2014