3200 Lakeside Dr.
Santa Clara , CA 95054
USA
Product Categories:
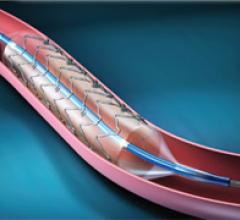
Embolic Protection Devices, Stents, Stents Drug Eluting, Vascular Closure Devices, Stents Carotid, Stents Peripheral, Stents Bare Metal
Embolic Protection Devices, Stents, Stents Drug Eluting, Vascular Closure Devices, Stents Carotid, Stents Peripheral, Stents Bare Metal


 July 14, 2011
July 14, 2011