August 9, 2017 — Abbott announced that the first patient has been enrolled in a clinical study to evaluate a minimally invasive clip-based repair system for treating people with moderate or severe tricuspid regurgitation (TR). The first patient was enrolled at Abbott Northwestern Hospital by Paul Sorajja, M.D., cardiologist at Minneapolis Heart Institute and Abbott Northwestern Hospital.
The transcatheter tricuspid valve repair (TTVR) system builds upon Abbott's MitraClip System, which has shown to predictably and effectively treat mitral regurgitation, a similar disease impacting the left side of the heart.
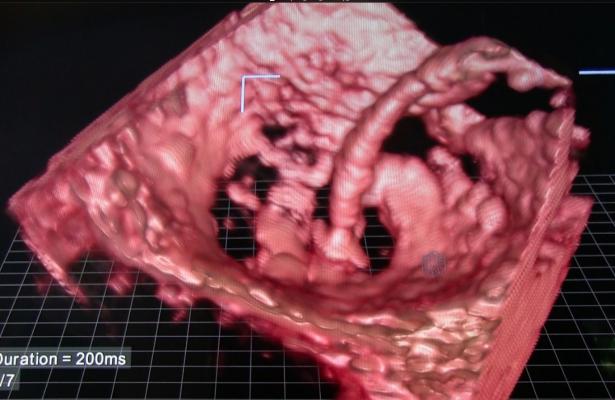
Tricuspid regurgitation is a condition in which the valve between the heart's two chambers on the right side does not close properly, resulting in a backward flow of blood into the right atrium. The consequences of leaving it untreated can be substantial – people often develop other conditions such as atrial fibrillation, heart failure and, ultimately, death. Currently, there are no approved minimally invasive treatments for people with moderate or severe tricuspid regurgitation.
"Current pharmacological and surgical treatment options are not meeting the needs of people living with tricuspid regurgitation," said Georg Nickenig, M.D., Ph.D., professor and chief, Department of Cardiology, University Hospital, Bonn, Germany, and lead investigator of the study. "Abbott's MitraClip has shown positive results for mitral regurgitation, and we hope this study shows that a similar clip-based technology may effectively treat people with tricuspid regurgitation."
The clinical trial is expected to support Abbott's application for CE Mark in Europe for a clip-based transcatheter tricuspid valve repair system.
The study, called TRILUMINATE, is a prospective, single-arm, multi-center study designed to evaluate the performance of clip-based technology in approximately 75 symptomatic patients at 25 sites across the United States and Europe. The primary endpoints are an echocardiographic tricuspid regurgitation reduction of ≥ 1 grade at 30 days post-procedure, and the assessment of major adverse events at six months.
For more information: www.mitraclip.com


 January 05, 2026
January 05, 2026 









