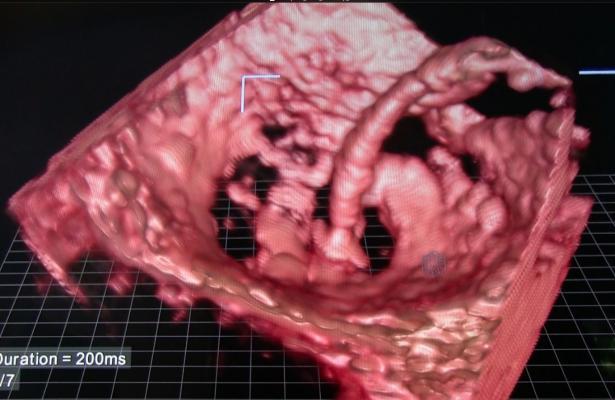
September 5, 2017 — Minneapolis Heart Institute Foundation announced it has enrolled the first-in-the-world patient in a clinical study to evaluate a minimally invasive clip-based repair system made by Abbott for treating people with moderate or severe tricuspid regurgitation (TR). This study is the first application of this minimally invasive technology for use in the tricuspid heart valve, where currently there are no options for most patients. Paul Sorajja, M.D., performed this first-in-human procedure at Minneapolis Heart Institute at Abbott Northwestern Hospital.
Tricuspid regurgitation is leakage of blood backwards into the right atrium through the tricuspid valve each time the right ventricle contracts. The increased volume of blood in the right atrium can cause it to enlarge, which can change the pressure in the nearby chambers and blood vessels. Currently there are no commercially available minimally invasive treatment options for people with moderate or severe TR and these patients face a poor prognosis.
The study, called TRILUMINATE, is a prospective, single-arm, multi-center study designed to evaluate the performance of clip-based technology in approximately 75 symptomatic patients at 25 sites across the United States and Europe. The primary endpoints are an echocardiographic tricuspid regurgitation reduction of ≥ 1 grade at 30 days post-procedure, and the assessment of major adverse events at six months.
“Patients who have tricuspid regurgitation have limited options for treatment. Current guidelines recommend medical therapies or invasive surgical options to treat this disease,” said Sorajja, director of the MHIF Valve Science Center and investigator in the TRILUMINATE study. “Since less invasive approaches are currently unavailable to treat TR, patients who meet these study criteria and are enrolled will have access to this innovative technology. The opportunity for MHIF to provide the first-in-the-world implant is unprecedented. The core mission of MHIF’s Valve Science Center is to improve the health of patients with valvular heart disease through clinical research, innovation and education. Through a multidisciplinary approach, MHIF continues to provide first-in-human trials that improve patient options and change cardiology care worldwide.”
Read the article "Abbott Initiates First Clinical Trial of Clip-Based Tricuspid Repair System"
For more information: www.mplsheart.org


 January 05, 2026
January 05, 2026 









