March 29, 2018 — Abbott announced the company has initiated the landmark GUIDE-HF clinical trial using the CardioMEMS HF System. The GUIDE-HF trial will study whether the CardioMEMS device can improve survival and quality of life for people living with New York Heart Association (NYHA) Class II - IV heart failure.
The system has already been proven, when managed by a physician, to significantly reduce heart failure hospital admissions and improve the quality of life for people living with NYHA Class III heart failure. Doctors use the NYHA classification system to classify heart failure according to the severity of a person's symptoms.
"Monitoring pulmonary artery pressure with Abbott's CardioMEMS device has already been shown to offer improvements in patient care. We now want to build a stronger body of clinical evidence, with GUIDE-HF, that establishes its role in improving patient survival," said JoAnn Lindenfeld, M.D., primary investigator for the GUIDE-HF trial and director of advanced heart failure at Vanderbilt University Medical Center in Nashville.
The prospective trial will enroll 3,600 patients at 140 hospitals across North America with stage C, NYHA Class II-IV heart failure with either elevated brain-type natriuretic peptide (BNP) levels or prior heart failure hospitalizations in the past 12 months.
First implants for the trial occurred recently at:
- Providence Hospital, Southfield, Mich., by Marcel Zughaib, M.D., and Herman Kado, M.D.;
- Sanford Medical Center, Sioux Falls, S.D., by Orvar Jonsson, M.D.; and
- Austin Heart, Austin, Texas by Kunjan Bhatt, M.D.
The GUIDE-HF trial is designed to build on the clinical experience gained from the CHAMPION trial and aims to provide additional clinical evidence to further expand coverage for this first-of-its-kind technology.
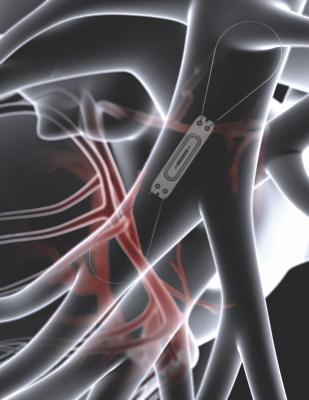
For people living with heart failure, changes in the pressure of blood through the pulmonary artery can indicate worsening heart failure — even before symptoms such as shortness of breath or weight gain are reported. Abbott's CardioMEMS HF System allows physicians to remotely monitor pressure changes before the patient's symptoms progress. This personalized approach allows physicians to more proactively manage a patient's care while reducing the likelihood of hospitalization.
The CardioMEMS HF System features a small pressure-sensing device, no larger than the size of a small paperclip, that is implanted through a minimally invasive procedure, directly into the patient's pulmonary artery. While at home, patients lay on a special pillow to wirelessly take a pressure reading. Data from the sensor is collected through radio frequency to the pillow's antenna and then is sent wirelessly to the patient's doctor. This information can then be used by physicians to proactively adjust medications and treatment plans, if needed.
For more information: www.sjm.com
Related CardioMEMS Content
New CardioMEMS Data Shows Effectiveness in Reducing Heart Failure Readmissions
CardioMEMS HF System Added to European Guidelines for Heart Failure Patients
VIDEO: Technologies to Reduce Heart Failure Readmissions


 January 05, 2026
January 05, 2026 









