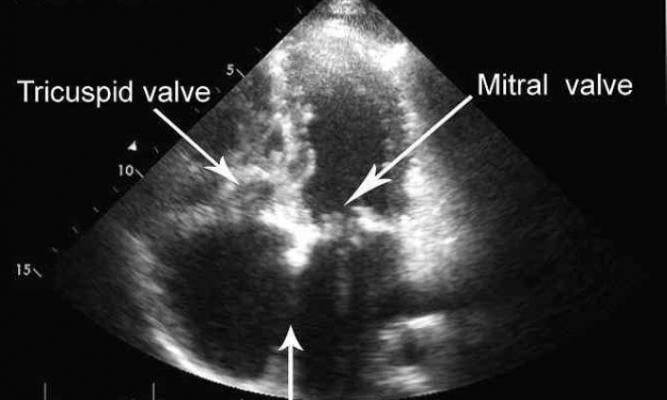
June 11, 2019 — Abbott recently announced positive late-breaking data from its TRILUMINATE study of the company's minimally invasive tricuspid valve repair system. Results at 30 days demonstrated the investigational device is associated with a reduction of tricuspid regurgitation (TR) symptoms — caused by a leaky tricuspid heart valve — suggesting a possible treatment option for people suffering from this difficult-to-manage structural heart disease. Abbott's transcatheter tricuspid valve repair (TTVR) system builds on the continued advances of the company's clip-based MitraClip technology, which treats people with leaky mitral valves.
Results from the TRILUMINATE study were presented in a late-breaking trial session at EuroPCR, the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EAPCI), May 21-24 in Paris, France.
"Treating a leaky tricuspid heart valve has long presented a significant challenge for cardiologists because it is an extremely complex and difficult heart valve to treat," said Georg Nickenig, M.D., Ph.D., professor and chief, Department of Cardiology, University Hospital, Bonn, Germany, and lead investigator of the study. "These data are extremely encouraging, and I am excited about the potential of transcatheter tricuspid valve repair as a minimally invasive treatment option for these very ill patients who have no other options."
Tricuspid regurgitation is a condition in which the valve between the two chambers on the right side of the heart does not close properly, resulting in a backward flow of blood into the right chamber of the heart, which can ultimately result in heart failure and death if left untreated.1, 2, 3 Approximately one in 30 people over the age of 65 in the U.S. alone have moderate or severe TR.4 Surgery is the only definitive treatment but is rarely performed due to the high mortality and morbidity rates associated with the procedure. Currently, there are no approved non-surgical, minimally invasive treatments for people with moderate or severe tricuspid regurgitation.
TRILUMINATE is a prospective, single arm, multi-center study at 21 sites in Europe and the United States. The study is evaluating the safety and performance of the tricuspid valve repair system in 85 patients with symptomatic moderate or greater tricuspid regurgitation who were determined to be good candidates for percutaneous transcatheter intervention. Results show that at 30 days:
-
Eighty-six-point-six (86.6) percent of patients saw a reduction in TR severity of at least one grade (p < 0.0001);
-
A significantly greater proportion of patients were categorized as NYHA class I or II (80.5 percent at 30 days, compared to 25.6 percent at baseline), an improvement that was statistically significant. Doctors use the New York Heart Association (NYHA) classification system (I-IV) to classify heart failure according to the severity of a person's symptoms, with class I causing little to no physical limitations and discomfort; and
-
Patients also experienced a mean improvement in KCCQ score — a self-assessment of social abilities, symptoms and quality of life — from 53.05 at baseline to 67.25 at 30 days, which was an increase of 14.20 points (a five-point increase is considered clinically significant).
Clinical follow-up will continue through six months and one, two and three years.
Abbott's transcatheter tricuspid valve repair system is an investigational device only.
For more information: www.abbott.com
Related TRILUMINATE Content
Abbott Initiates First Clinical Trial of Clip-Based Tricuspid Repair System
VIDEO: Transcatheter Tricuspid Valve Repair and Replacement Technologies
VIDEO: Tricuspid Device Clinical Trial Overview
References
1. Singh J.P. , Evans J.C. , Levy D., et al . Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897–902.doi:10.1016/S0002-9149(98)01064-9.
2. Vassileva C.M. , Shabosky J., Boley T., et al . Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg 2012;143:1043–9.doi:10.1016/j.jtcvs.2011.07.004.
3. Stuge O., Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg 2006;132:1258–61.doi:10.1016/j.jtcvs.2006.08.049.
4. Topilski Y. Tricuspid valve regurgitation: epidemiology and pathophysiology. Minerva Cardioangiologica 2018 Mar 28 DOI: 10.23736/S0026-4725.18.04670-4).


 January 05, 2026
January 05, 2026 









