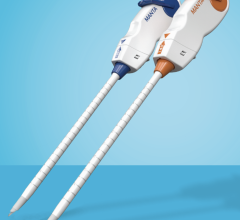
May 29, 2007 — The FDA has cleared the Mynx VCS vascular closure system (VCD) by AccessClosure designed to seal a puncture site in the femoral artery and stop the bleeding after a cardiac catheterization procedure.
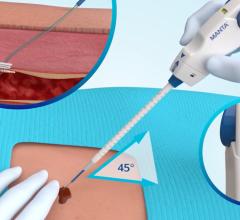
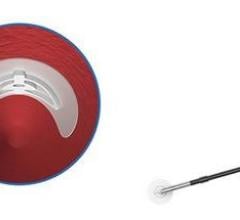
The Mynx VCS uses a balloon catheter and a standard procedural sheath to deliver an extravascular, hydrogel sealant used to seal the puncture site. Following the cardiac catheterization procedure, the Mynx VCS balloon catheter is inserted through the introducer sheath into the femoral artery to temporarily stop bleeding at the puncture site.
The hydrogel sealant is injected through the introducer sheath at the puncture site and within the tissue tract that stops the bleeding and seals the access site. Once sealed, the balloon catheter is deflated and removed along with the introducer sheath and manual compression is applied for one to two minutes to ensure bleeding stops. The gel will absorb into the body within 30 days.
The Mynx is used on patients who have undergone diagnostic or interventional endovascular procedures, and is indicated for use to seal femoral arterial access sites while reducing times to hemostasis and ambulation utilizing a 5F, 6F or 7F procedural sheath.
For more information visit www.fda.gov/cdrh/pdf4/p040044s001.html, and www.fda.gov/hearthealth.

 October 07, 2025
October 07, 2025