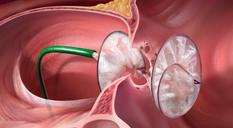
The Gore Helex septal occluder.
August 25, 2010 – Alleging infringement of a patent for its transcatheter cardiac septal occluder devices, AGA Medical Holdings Inc. said it filed a lawsuit against W.L. Gore & Associates.
AGA alleges Gore is infringing its U.S. Patent No. 5,994,738, which covers certain Amplatzer transcatheter occlusion products for the treatment of structural heart defects. AGA is seeking damages against Gore and a permanent injunction prohibiting them from manufacturing and selling the Gore Helex Septal Occluder both in the United States and abroad.
AGA Amplatzer occlusion devices offer minimally invasive, transcatheter treatments in cardiac defect closure. AGA has occlusion devices approved to close seven different structural heart defects.
The company has been a market leader in this field, but its market share has been seriously challenged by Gore’s Helex device.
For more information: www.amplatzer.com, www.goremedical.com


 June 20, 2024
June 20, 2024 









