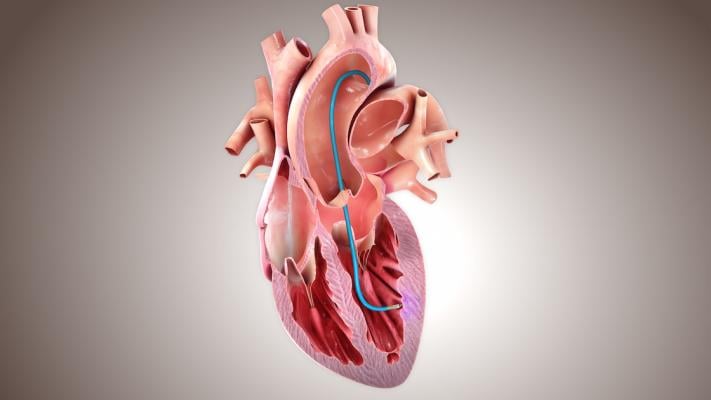
BioCardia's CardiAMP cell therapy uses a patient’s own (autologous) bone marrow cells delivered to the heart in a minimally invasive, catheter-based procedure to potentially stimulate the body’s natural healing response. The therapy incorporates a pre-procedural screening assay to identify those patients who are likely responders to the therapy, a first for a cardiac cell therapy.
October 28, 2021 – The first patient has been treated in a new trial using stem cell therapy to treat chronic myocardial ischemia with refractory angina. BioCardia Inc., a company focused on developing cellular and cell derived therapeutics for the treatment of cardiovascular and pulmonary diseases, announced the treatment of the first patient in its Phase III pivotal CardiAMP Cell Therapy Chronic Myocardial Ischemia Trial for patients with no other options.
These patients experience frequent angina attacks that are uncontrolled by optimal drug therapy but are not suitable candidates for stent placement or bypass surgery, leaving them with no therapeutic options.
The first patient was treated with the investigational CardiAMP autologous cell therapy at the University of Wisconsin in Madison, by Amish Raval, M.D., an interventional cardiologist and associate professor of medicine, supported by Peiman Hematti, M.D., bone marrow transplantation hematologist and professor of medicine.
The CardiAMP Cell Therapy Chronic Myocardial Ischemia Trial is expected to enroll up to 343 patients at up to 40 centers. The primary endpoint will evaluate improvement in exercise tolerance at six months following the study procedure. The trial is intended to have an adaptive statistical analysis plan with an initial assessment for efficacy when 100 patients reach their primary endpoint, although aspects of the statistical analysis plan remain the subject of study considerations with the FDA.
The co-National Principal Investigators for the trial are Thomas Povsic, M.D., of Duke University, Durham, NC, and Timothy Henry, M.D., of The Christ Hospital, Cincinnati, OH.
CardiAMP Cell Therapy uses a patient’s own (autologous) bone marrow cells delivered to the heart in a minimally invasive, catheter-based procedure to potentially stimulate the body’s natural healing response. The therapy incorporates a pre-procedural screening assay to identify those patients who are likely responders to the therapy, a first for a cardiac cell therapy.
Patients eligible to undergo the cell therapy procedure receive cells delivered with a system that has been shown in published literature to present the lowest risk to patients for biotherapeutic delivery[1] and to be three to six times more efficient at delivering cells to the heart muscle than other methods.[2] This approach allows the patient to be discharged from the hospital the morning after the study procedure.
The executive steering committee for this pivotal study includes distinguished physician scientists, in addition to the co-National Principal Investigators include:
• Amish Raval, M.D., University of Wisconsin, Madison, WI
• Carl Pepine, M.D., University of Florida, Gainesville, FL
• Bernard J. Gersh, M.B., Ch.B., D.Phil., Mayo School of Medicine, Rochester, MN
Raval and Pepine are co-national principal investigators for the ongoing CardiAMP Cell Therapy Heart Failure Trial that paved the way for the new chronic myocardial ischemia trial.
Earlier this year, the FDA approved a detailed protocol amendment to shorten the trial’s primary endpoint to a six-month follow-up from one year, and to harmonize details of the protocol to correspond with the actively enrolling CardiAMP Cell Therapy Heart Failure Trial, which has incorporated best practices from significant interactions with study centers and the FDA. Both trials have a Category B designation from FDA and the Center for Medicare and Medicaid Services (CMS), enabling study sites to receive CMS reimbursement for both the standard of care procedures and the investigational CardiAMP Cell Therapy System.
“This is an important therapeutic development effort that has the potential to meaningfully benefit patients suffering from refractory angina due to chronic myocardial ischemia,” said BioCardia CEO Peter Altman, Ph.D. “We expect this trial to stand on its own as an independent program with a high probability of producing the primary safety and efficacy data to support FDA approval for the CardiAMP Cell Therapy for these angina patients. Furthermore, we expect this trial enrolling concurrent with the CardiAMP Cell Therapy Heart Failure Study may help accelerate enrollment with the former, while also building a larger body of evidence on the CardiAMP cell therapy system.”
About Chronic Myocardial Ischemia with Refractory Angina
Despite improvements in revascularization techniques, there is a growing population of patients with chronic angina who have severely limiting symptoms but are not candidates for further revascularization. Recent studies suggest that five to 15 percent of patients undergoing cardiac catheterization have significant disease that cannot be treated with revascularization.[3,4] It is estimated that between 600,000 and 1.8 million U.S. patients suffer from refractory angina, with approximately 75,000 new cases diagnosed each year.[5]
These patients experience poor perceived health status and psychological distress,[6] have significant impairments in quality of life,[7] and represent a financial burden to the healthcare system.[8] Ranolazine and enhanced external counter pulsation are the only contemporary therapies developed for the treatment of this condition; however, recent studies have highlighted the limitations of Ranolazine,[9] while enhanced counter pulsation has been associated with minimal reduction in angina.[10]
About CardiAMP Bone Marrow Derived Stem Cell Therapy
BioCardia developed of two biotherapeutic platforms – the CardiAMP autologous bone marrow derived mononuclear cell therapy for cardiovascular indications, and the NK1R+ allogenic bone marrow derived mesenchymal stem cell therapies for cardiovascular and pulmonary diseases. These platforms underly four product candidates, each with the potential to meaningfully benefit millions of patients, the company said. Three of these investigational therapies are enabled by the company’s biotherapeutic delivery platforms, which BioCardia also selectively licenses to other biotherapeutic development firms.
References:


 November 19, 2021
November 19, 2021 









