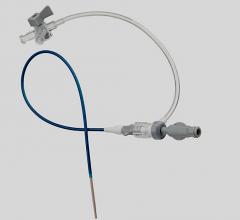
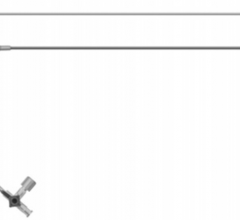
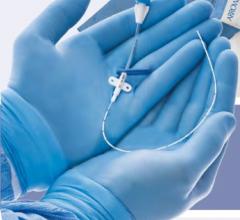
March 31, 2014 — BioCardia Inc. announced it received CE mark for its Morph AccessPro steerable introducer, designed for easier navigation through the vasculature during delivery of biotherapeutics and medical devices.
The steerable thin-walled catheter shaft technology provides a flexible pathway for biologic delivery systems, balloon dilatation catheters, stents, guidewires and micro infusion systems. The unique introducer is designed to enhance physician control and reduce complications and catheter exchanges, minimizing procedure time and radiation exposure. It will be commercially available in the European Union in the coming months.
“Many times, the primary reason for failure of a particular procedure is the inability to gain access into the aortic branches with a guide catheter or guide sheath,” said Rodney S. Badger, M.D., chief of interventional cardiology and associate professor of medicine for the University of Utah. “This introducer is valuable today to improve access for aorto-ostial procedures, thereby enhancing safety and shortening procedure times. It also has future potential to enhance biotherapeutic interventions for diseases of the organs fed by these arteries, such as inflammatory bowel diseases, renal diseases and even diseases involving the pancreas.”
Current commercially available guide catheters have pre-formed distal curves shaped like a “hockey stick” or a “shepherd’s crook,” designed to maneuver within specific vascular anatomical configurations. If a patient’s anatomy is complicated and does not match the catheter shape, access can be time-consuming and difficult to achieve.
The Morph AccessPro enables a physician to customize the catheter shape to an individual patient’s anatomy, allowing access to the contra-lateral superficial femoral artery (SFA) all the way down to the trifurcation, the renal arteries and the mesenteric arteries.
The Morph AccessPro is currently being used commercially in 350 U.S. hospitals and has been used in thousands of cases to date.
For more information: www.biocardia.com

 June 10, 2020
June 10, 2020