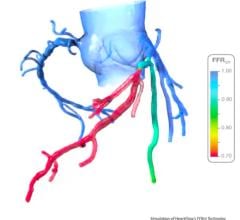
September 27, 2023 — Boston Scientific announced the FDA clearance of the AVVIGOTM+ Multi-Modality Guidance System, a next-generation intravascular ultrasound (IVUS)1 and fractional flow reserve (FFR)2 system with advanced software and hardware features designed to provide high-quality IVUS vessel imaging and physiology experience during percutaneous coronary intervention (PCI) procedures. Building upon the AVVIGO Guidance System II, this technology helps to inform treatment decisions and enable faster, more efficient treatment procedures such as angioplasties3 and atherectomies for patients with coronary artery disease. The company also recently received CE Mark, with launch anticipated early next year.
Key features of the AVVIGO+ system include:
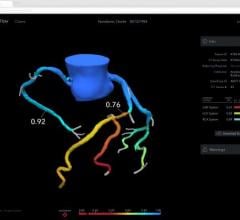
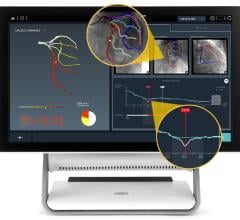
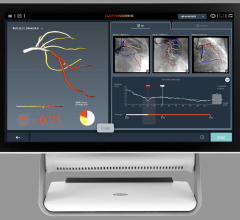
- First IVUS system with an artificial intelligence (AI) software called Automated Lesion Assessment to automate key procedural steps and provide precise vessel measurements
- Reduces procedure time significantly by acquiring IVUS images at a faster speed
- Provides enhanced guidance by drawing a physiology graph that helps to provide a roadmap to treat the diseased coronary artery
The American Heart Association and American College of Cardiology Committee on Clinical Practice Guidelines now recommend4 intracoronary imaging as routine clinical practice in PCI procedures.
“We are pleased to introduce U.S. clinicians to the next-generation AVVIGO+ Multi-Modality Guidance System, which builds upon the AVVIGO Guidance System II and provides fast, intuitive and accurate vessel and lesion assessment capabilities for percutaneous coronary interventions,” said Lance Bates, president, Interventional Cardiology Therapies, Boston Scientific. “Coming on the heels of the updated ACC guidelines recommending intracoronary imaging during PCIs, we believe this enhanced and automated tool will help physicians optimize these procedures to provide better outcomes for their patients with coronary artery disease.”
For more information: www.bostonscientific.com
References:
1 Intravascular Ultrasound (IVUS) is an imaging technique that provides physicians with a detailed look into the blood vessels of the heart, from the inside-out, which can provide additional information about the appropriate stent-size required to treat the patient. Once the stent has been placed inside the blood vessel, the inside view of the blood vessel through IVUS can also help determine if the stent is covering the diseased area completely and properly hugging the vessel wall.
2 Fractional flow reserve (FFR) is a minimally invasive procedure to determine the severity of the narrowing (stenosis) inside the coronary arteries. FFR measures blood pressure differences across a coronary artery stenosis to determine the likelihood that the stenosis impedes oxygen delivery to the heart muscle. The FFR measurement allows the physician to determine if PCI is necessary.
3 One of the common ways to treat CAD is by performing an angioplasty. During the procedure, a thin tube known as a guide catheter is inserted into the artery at the groin or wrist. A small balloon located at the end of a second catheter is moved through the guide catheter to the site of the narrowing. The balloon is then inflated to reduce the blockage. The balloon is deflated and removed. Then a drug-eluting stent mounted on another catheter is implanted to help keep the artery open.
4 Truesdell A, Alasnag M, Kaul P, et al. Intravascular Imaging During Percutaneous Coronary Intervention. J Am Coll Cardiol. 2023 Feb, 81 (6) 590–605


 February 03, 2026
February 03, 2026