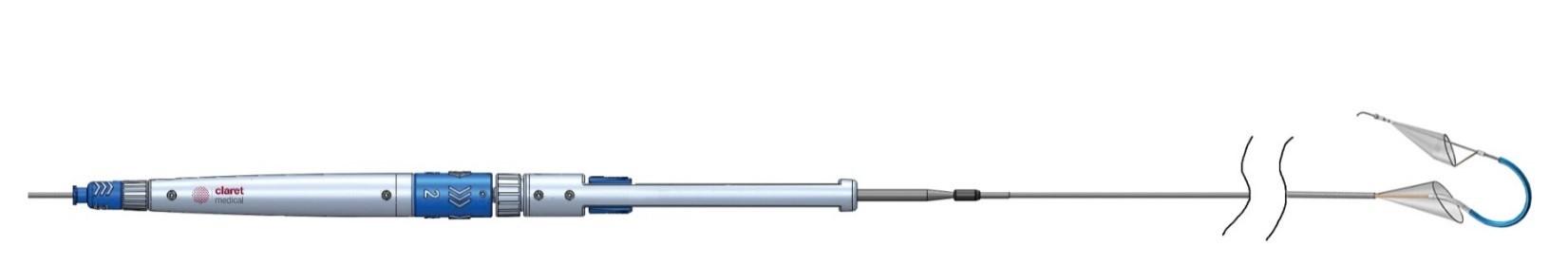
January 28, 2014 — Claret Medical Inc. received CE marking for the Sentinel Cerebral Protection System (CPS) for
embolic protection during transcatheter aortic valve replacement (TAVR).
The Sentinel CPS features vessel apposition benefits and is a filter-based system that captures and removes embolic debris that is released and travels to the brain during TAVR procedures.
"Cerebrovascular events remain a major concern during TAVR due to the macroscopic material that is liberated during the procedure from
catheter manipulation as well as from the burden of atheroma in the vasculature and the native valve," said Dr. Nicholas Van Mieghem, Department of Interventional Cardiology, Erasmus Medical Center, Rotterdam, The Netherlands. "Other devices deflect debris downstream but do not remove it from the circulation. Since studies have shown that embolic debris traveling to the brain can be captured in more than 75 percent of procedures when a filter is used, embolic protection could become the standard of care in TAVR."
In the United States, the Sentinel CPS will be evaluated under an investigational device exemption (IDE) in a multicenter pivotal
study involving up to 15 centers in the United States and Europe.
Claret Medical is currently focusing developmental and clinical research resources on addressing the problem of stroke during TAVR.
For more information: www.claretmedical.com