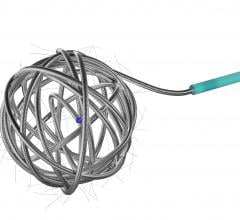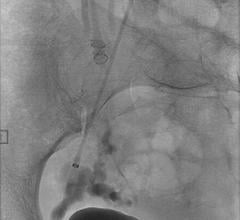
April 29, 2013 — Cook Medical has launched a new fully-retractable .035 inch embolization coil, intended for peripheral arterial and venous embolization. Embolization is a nonsurgical, minimally invasive procedure performed by a physician to block or reduce blood flow in arteries and veins. Cook showcased the Retracta Detachable Embolization Coil at the annual Society of Interventional Radiology (SIR) meeting.
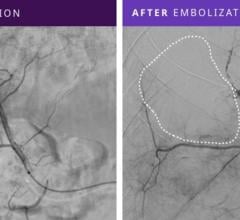
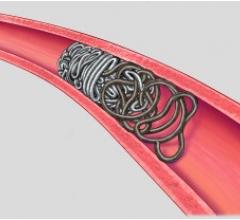
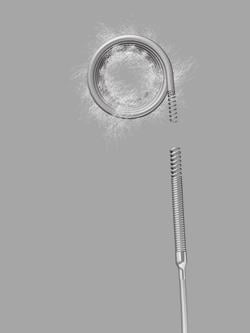
The new Retracta coil is designed to provide the physician with more controlled delivery of embolization coils. During this procedure, a physician places a coil that forms within the vessel and causes a clot to form, which may stop or can slow the blood flow. With the Retracta system, the physician can place the coil and detach it with precision when ready. Visibility of the detachment zone allows a clinician to easily identify when the Retracta is ready to be detached. If the physician is not satisfied with the positioning of the coil in the vessel, he/she can fully retract the coil into the catheter and reposition it before detachment.
“When trying to control bleeding, precise coil placement is critical in embolization procedures,” said Andrew Conder, senior global product manager for embolization at Cook Medical. “The Retracta is simple and affordable, and gives physicians control over placement.”
Based on the construction of Cook Medical's Nester coils, the Retracta coils use platinum technology to allow soft packing of the coil. Physicians can confirm placement accuracy under fluoroscopy by seeing the change in radiopacity at the detachment zone. If the physician wishes, he/she can inject a contrast medium to confirm that the coil is correctly placed. Like the Nester coil, the Retracta can be used right out of the package.
For more information: www.cookmedical.com


 June 05, 2025
June 05, 2025