September 29, 2009 – The United States Court of Appeals for the Federal Circuit last week affirmed the previous ruling of the United States District Court for the Northern District of California that Cook Incorporated does not infringe on Edwards’ patents for endovascular devices to treat aneurysms, Cook officials said.
“Since 2003, when this case began, we have remained steadfast that the claims were without merit, and we aggressively defended this case on that basis,” said Cynthia Kretz, general counsel for Cook. The company was represented by the law firm of Brinks Hofer Gilson & Lione in the litigation.
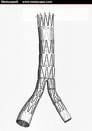
The original case, C 03-03817 JSW, involved Edwards claiming patent infringement on four of Cook’s Zenith endovascular stent-grafts for use in treating abdominal aortic aneurysms (AAAs) and thoracic aortic aneurysms (TAAs).
The United States Court of Appeals for the Federal Circuit reaffirmed the District Court’s ruling “that no reasonable jury could find infringement” or “conclude that Cook’s accused devices” literally infringed the Edwards patents, and “that Edwards cannot, as a matter of law, show that Cook’s accused devices infringe the patents-in-suit under the doctrine of equivalents. Cook, therefore, is entitled to judgment in its favor on this basis as well,” the court wrote in its original decision.
For more information: www.cookmedical.com


 April 26, 2023
April 26, 2023 









