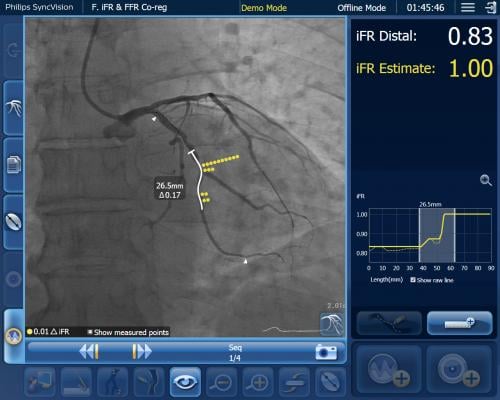
Philip's co-registration technology showing angiography overlaid with iFR in a coronary vessel segment with a system of dots that show regions where the hemodynamic pressure drops occur. This can aid in identifying culprit lesions.
October 15, 2020 — The one-year results of the late-breaking DEFINE PCI study showed patients had improved outcomes and less recurrent chest pain at one year using instant wave-Free Ratio (iFR) physiologic measurements.[1] The one-year data showed an optimal post-PCI iFR of ≥0.95 was associated with improved event-free survival. In fact, patients with a post-PCI iFR of ≥0.95 had 68% fewer clinical events than patients with less than optimal post-PCI iFR values (1.8% vs 5.7%, p=0.04).
The data were presented at the Transcatheter Cardiovascular therapeutics (TCT) Connect 2020 virtual conference. The study assessed the level of residual ischemia in patients after a percutaneous coronary intervention (PCI) using a blinded instant wave-Free Ratio (iFR) pullback measurement, a physiologic guidance technology developed by Philips Healthcare.
The exciting promise of this data is that by using iFR co-registration physicians can identify precise locations causing ischemia, plan stent length and even place a virtual stent to predict physiologic improvement before the intervention is performed. The blinded acute data of DEFINE PCI revealed that 24% of patients with angiographically successful PCI still had residual ischemia. In about 82% of these patients, the residual ischemia was the result of a focal, potentially treatable lesion. The data suggests patients could have benefited from planning tools like iFR co-registration to find potentially treatable lesions that are often not identified by angiography alone.
The DEFINE PCI one-year data release shows that patients whose baseline ischemia was more effectively treated (post-PCI iFR ≥0.95) had improved outcomes and less recurrent angina (chest pain) at one year. The one-year data showed an optimal post-PCI iFR of ≥0.95 was associated with improved event-free survival. In fact, patients with a post-PCI iFR of ≥0.95 had 68% fewer clinical events than patients with less than optimal post-PCI iFR values (1.8% vs 5.7%, p=0.04).
“In DEFINE PCI we noted that if all lesions causing focal ischemia had been treated up-front, the rate of significant ischemia could theoretically be reduced from 24% to 5%,” said Allen Jeremias M.D., principal investigator of the DEFINE PCI study. “Now with the one-year data, we find that patients with more complete resolution of ischemia do better clinically. To some that may not be a surprising finding, but we are conducting the science because, today, most interventionalists are only using physiology as a “who-to-treat” tool. Beyond who we should treat, tools like iFR can guide “how” and “where” to treat within a vessel and then confirm results after stent placement. It’s difficult to know which lesions will produce a significant physiological gradient and which won’t. If you don’t measure, really there’s no way of telling.”
"We now have technology such as iFR co-registration to determine the risk-benefit of revascularization as well as when, how, and where we should treat – and that should lead to better outcomes for patients. “The goal is to get patients as close to normal, physiologically, as possible,” said Manesh Patel, M.D., co-author of the DEFINE PCI study. “We’ve known this for a while, but we haven’t had the mature technology to deliver on this. We now have technology such as iFR co-registration to determine the risk-benefit of revascularization as well as when, how, and where we should treat – and that should lead to better outcomes for patients.”
The DEFINE PCI one-year data release follows the 500 patients who participated in the DEFINE PCI study announced last year at the American College of Cardiology (ACC) annual conference. The initial study results showed that 1 in 4 patients treated with standard of care PCI left the cath lab with residual ischemia (iFR < 0.90).[1]
“The one-year data from DEFINE PCI are the latest evidence that iFR contributes to reduced costs, enhanced patient experience and improved outcomes for PCI procedures [2,3,4],” said Chris Landon, Senior Vice President and General Manager Image Guided Therapy Devices at Philips. “With the recent introduction of OmniWire, the world’s first solid core pressure guide wire for coronary artery interventional procedures, as well as the next generation of our Azurion platform, we are advancing image-guided therapy with innovative, procedure-focused solutions.”
DEFINE GPS Study Evaluating if iFR Coregistration Improves Outcomes in a Large, Prospective, Randomized trial
In February, Philips announced DEFINE GPS, a randomized, controlled prospective trial which represents the next step in the DEFINE series of physiology studies. DEFINE GPS will assess the clinical effectiveness of iFR co-registration guidance to minimize post-PCI ischemia in patients. The study, which will include up to 3,000 participants at approximately 100 sites globally, will help determine whether a physiology-based PCI approach results in superior patient outcomes compared to standard angioplasty. Enrolment is expected to begin in Q1 2021.
The DEFINE PCI and DEFINE GPS studies are sponsored by Philips with the Cardiovascular Research Foundation overseeing core lab and clinical event committee activities.
Related iFR Content:
DEFINE GPS Study Assesses PCI Guided by Integrated iFR and Angiography
Updated SCAI Expert Consensus Says Either FFR, iFR Can Be Used
VIDEO: iFR Equal to FFR Outcomes in Coronary Lesion Evaluation — Interview with Justin Davies, MBBS, and Matthias Götberg, M.D.
New Technology Directions in Fractional Flow Reserve (FFR)
iFR More Cost-Effective Than FFR in PCI Guidance, DAIC
Easier to Use iFR Equal to Outcomes of FFR in Coronary Lesion Evaluation
VIDEO: iFR Found More Cost Effective Over Standard FFR — Interview with Manesh Patel, M.D.
One in Four Patients Have Residual Ischemia Following PCI
References:


 November 14, 2025
November 14, 2025 









