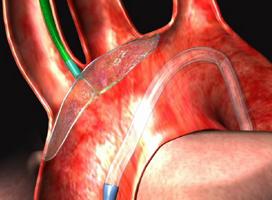
March 14, 2011 – Expanding its transcatheter valve accessories with the addition of an aortic embolic protection device, Edwards Lifesciences Corp. completed the acquisition of Embrella Cardiovascular Inc. The company developed the Embrella Embolic Deflector System, which can be used during transcatheter heart valve procedures.
The purchase price was about $43 million cash.
"This acquisition is consistent with our dedication to the ongoing exploration and development of novel technologies that have the potential to improve outcomes for patients, and supports our strategy to extend Edwards' global leadership in transcatheter heart valves," said Larry L. Wood, Edwards' corporate vice president, transcatheter valve replacement.
The single-use, disposable Embrella device is placed in the aorta through a sheath inserted in the right brachial or right radial artery. Its porous membrane allows blood flow to the brain while simultaneously deflecting embolic material. The Embrella Embolic Deflector System has been assessed in a limited clinical study in Europe and received CE mark there in May 2010.
For more information: www.edwards.com


 April 25, 2023
April 25, 2023 









