December 7, 2017 — Edwards Lifesciences Corp. announced the acquisition of Harpoon Medical Inc., a privately held medical technology company pioneering beating-heart repair for degenerative mitral regurgitation (DMR). Edwards announced a structured upfront investment in Harpoon in 2015, with an exclusive option to acquire the company.
Under the terms of the merger agreement, Edwards paid $100 million in cash for Harpoon at closing on Dec. 1. In addition, there is the potential for up to $150 million in pre-specified milestone-driven payments over the next 10 years.
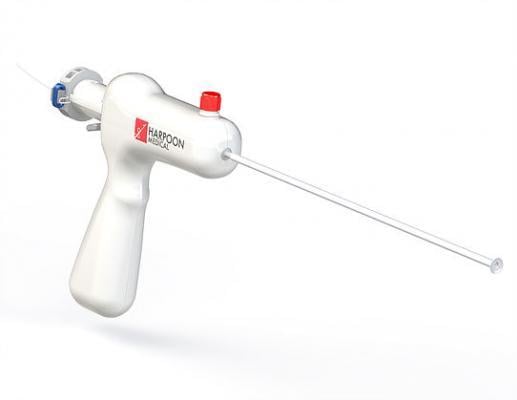
The Harpoon system is designed to facilitate echocardiography-guided repair of mitral valve regurgitation, by stabilizing the prolapsed mitral valve leaflet to restore proper coaptation and valve function. The Harpoon device is currently investigational and not available for commercial use. It is expected to receive CE Mark approval soon.
"There are a significant number of patients currently undergoing mitral valve surgery that we believe can benefit from Harpoon's therapy during a minimally invasive, beating-heart procedure," said device inventor James S. Gammie, M.D., chairman of the company's scientific advisory board and professor and chief of cardiac surgery at the University of Maryland School of Medicine. "This therapy offers the potential for earlier treatment of degenerative mitral valve disease with faster recovery and less morbidity, while also providing the opportunity for more consistent procedures and outcomes for patients."
Read the article "Harpoon Mitral Valve Repair System Found Safe and Effective."
Read the article "Advances and Future Directions for Transcatheter Valves."
For more information: www.edwards.com, www.harpoonmedical.com


 November 14, 2025
November 14, 2025 









